Novel FLT3 kinase inhibitor and synthesis and application thereof
A kinase inhibitor and a novel technology, applied in the field of novel FLT3 kinase inhibitor and its synthesis and application, can solve problems such as inability to cope with intensive chemotherapy regimens, and achieve the effects of low price and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
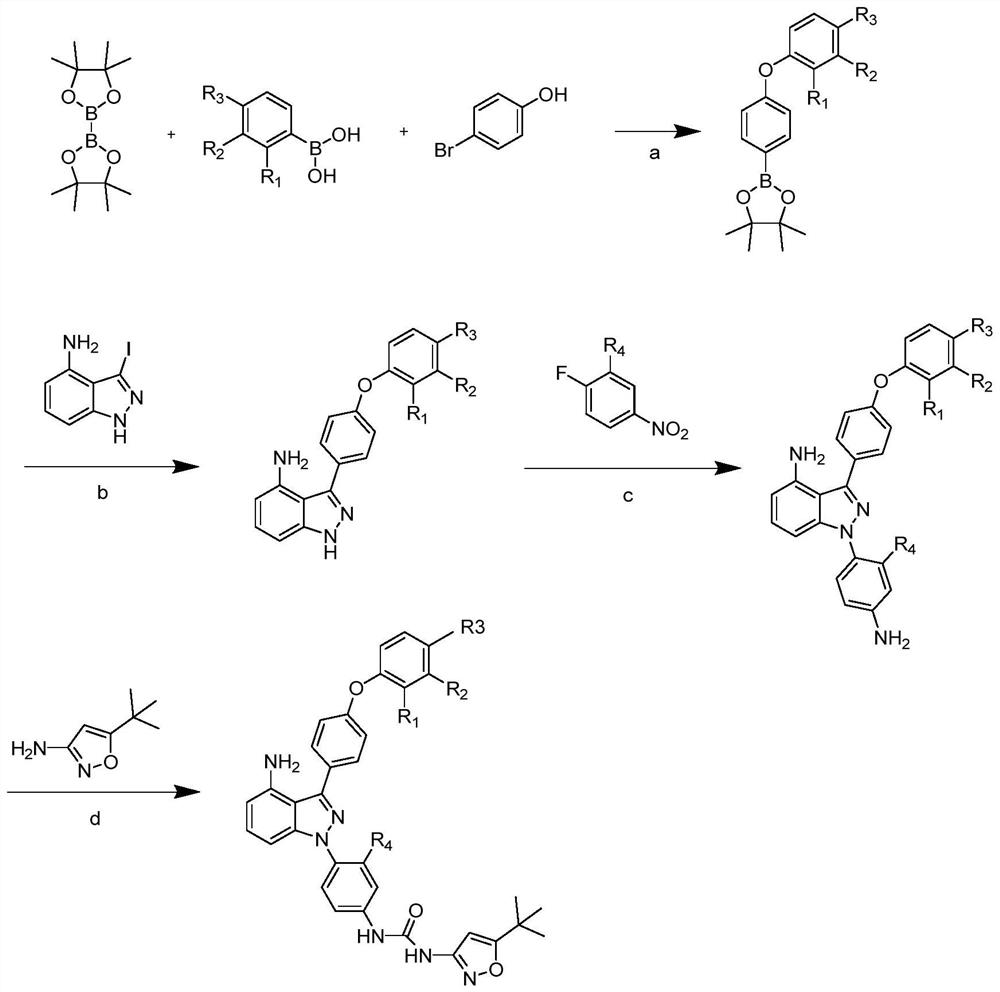
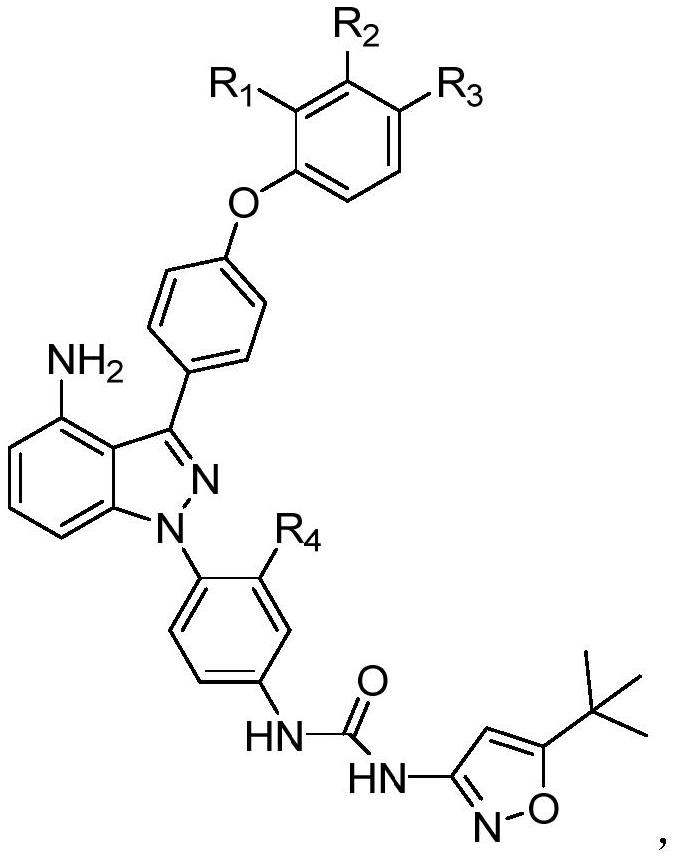
[0067] The name of the compound synthesized in this example is 1-(4-(4-amino-3-(4-(m-tolyloxy)phenyl)-1H-indazol-1-yl)-3-methylphenyl) -3-(5-(tert-butyl)isoxazol-3-yl)urea, its structural formula is as follows:
[0068]
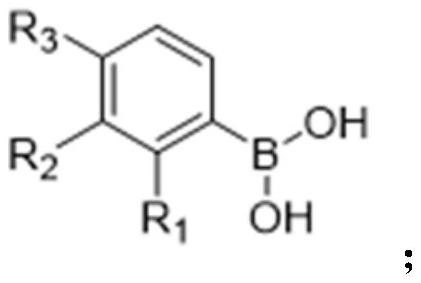
[0069] Step a: (7g, 40.46mmol) p-bromophenol, (5.43g, 44.51mmol) 3-methylphenylboronic acid and (10g, 39.38mmol) diboronic acid pinacol ester (divaleroyl diboron) were added to 100ml Add (8.08g, 44.51mmol) copper acetate and (4.91g, 48.55mmol) triethylamine to 1,2-dichloroethane solvent, and react at room temperature under nitrogen protection for 24h; the reaction mixture is filtered through diatomaceous earth. The filtrate was concentrated in vacuo to give a residue, which was purified by chromatography on silica gel, eluting with 5% by volume ethyl acetate (EtOAc) in hexanes. (12g, 122.27mmol) Potassium acetate, (2.96g, 4.05mmol) 72287-26-4 (i.e. 1,1'-bisdiphenylphosphinoferrocenepalladium dichloride (PdCl 2 (dppf))), solvent 100ml DMF, 15h, 100°C (rea...
Embodiment 2
[0081] The name of the compound synthesized in this example is 1-(4-(4-amino-3-(4-(3-isopropylphenoxy)phenyl)-1H-indazol-1-yl)phenyl)- 3-(5-(tert-butyl)isoxazol-3-yl)urea, its structural formula is:
[0082]
[0083] Step a: Add (5.8g33.52mmol) p-bromophenol, (6g 36.88mmol) 3-isopropylphenylboronic acid and (9.36g, 36.88mmol) biboronic acid pinacol ester to 100ml 1,2-dichloroethyl Add appropriate amount of (6.7g, 36.88mmol) copper acetate and (4g, 40.23mmol) triethylamine to the alkane solvent, and react at room temperature under nitrogen protection for 24h; the reaction mixture is filtered through diatomaceous earth. The filtrate was concentrated in vacuo to give a residue which was purified by silica gel chromatography eluting with 5% by volume EtOAc in hexanes. (9.87g, 100.57mmol) potassium acetate, (3g, 4.21mmol) 72287-26-4, solvent 100ml DMF, 15h, 100°C. The mixture was then passed through a short column of silica gel to remove insoluble material. The solvent was th...
Embodiment 3
[0088] The name of the compound synthesized in this example is 1-(4-(4-amino-3-(4-(m-tolyloxy)phenyl)-1H-indazol-1-yl)-3-methylphenyl) -3-(5-(tert-butyl)isoxazol-3-yl)urea, its structural formula is:
[0089]
[0090] Step a: 7g of p-bromophenol, 6g of 3-methylphenylboronic acid and 11.3g of pinacol diborate were added to 100ml of 1,2-dichloroethane solvent, and then an appropriate amount of copper acetate and triethylamine were added. Under the protection of nitrogen, the reaction was carried out at room temperature for 24 hours; the reaction mixture was filtered through diatomaceous earth. The filtrate was concentrated in vacuo to give a residue which was purified by silica gel chromatography eluting with 5% by volume EtOAc in hexanes. 11.91g potassium acetate, 2.98g 72287-26-4, solvent 100ml DMF, 15h, 100°C. The mixture was then passed through a short column of silica gel to remove insoluble material. The solvent was then removed under reduced pressure to afford a cru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com