Alanine-glyoxylate transaminase mutant with improved enzyme activity and application thereof
A technology of glyoxylate transaminase and alanine, which is applied in applications, nucleic acid carriers, enzymes, etc., can solve the problems of production and application that cannot meet the industrial scale and low output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
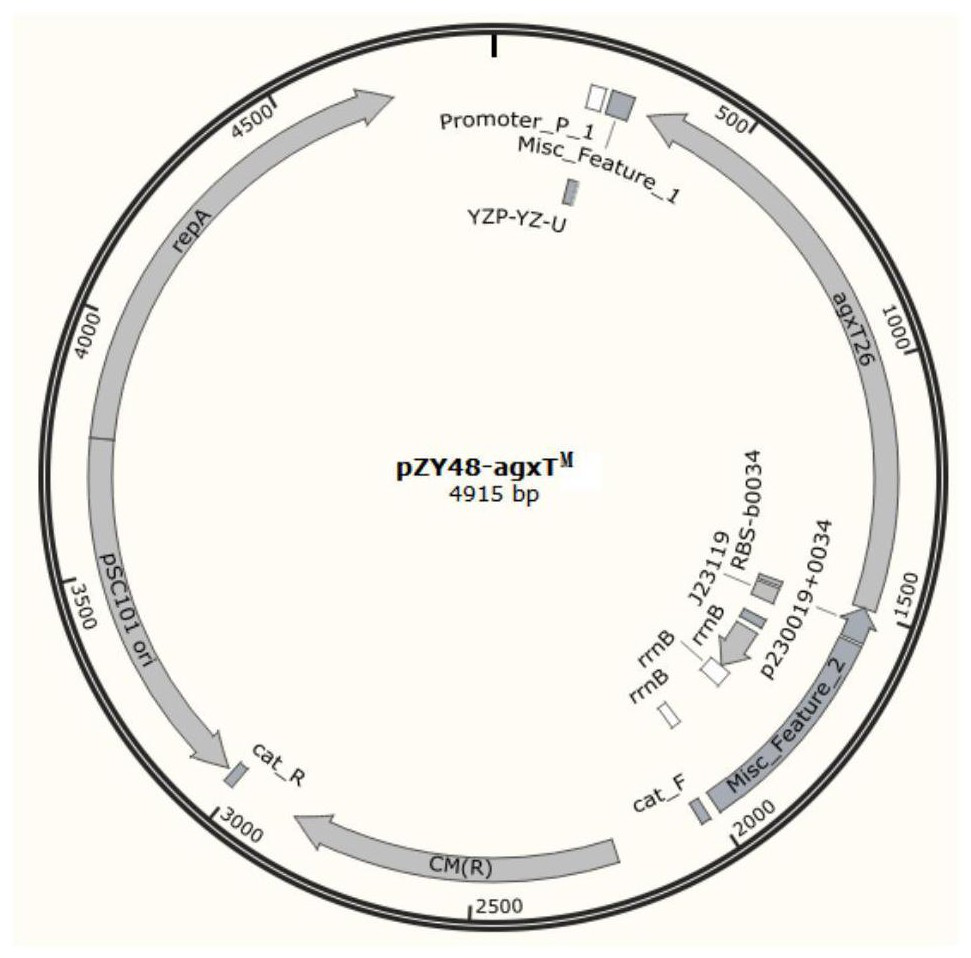
[0049] pZY48-agxT M Plasmid construction:
[0050] get agxT M Fragment: agxT-F shown in SEQ ID No.5 and agxT-R shown in SEQ ID No.6 as primers, pUC18-agxT shown in SEQ ID No.12 M As a template, agxT shown in SEQ ID No.3 is obtained by PCR method M Fragment;
[0051] Obtain pZY48 fragment: with pZY48-F shown in SEQ ID No.7 and pZY48-R shown in SEQ ID No.8 as primers, with plasmid pZY48 as template (source is Addgene (https: / / www.addgene.org / )), amplified by PCR to obtain the pZY48 fragment shown in SEQ ID No.13;
[0052] Construct pZY48-agxT M Plasmid: agxT will be obtained with the above steps M The pZY48 fragment was ligated with the CPEC method (Quan, J., & Tian, J..(2014). Methods in Molecular Biology, 1116, 103.) to obtain a plasmid named pZY48-agxT M , as shown in SEQ ID No.9, the schematic diagram of the plasmid is figure 1 ;
[0053] Host cell Escherichia coli MG1655-pZY48-agxT carrying mutant gene or mutant enzyme of alanine-glyoxylate aminotransferase M C...
Embodiment 2
[0058] Alanine-glyoxylate aminotransferase activity assay:
[0059] Take control strains MG1655-pZY48-agxT and MG1655-pZY48-agxT M Put the monoclonal strain into fresh LB liquid medium and culture it to OD at 37°C and 220rpm 600 Stop culture when it is 0.6. Take 8 mL of bacterial liquid at 12000 rpm, centrifuge at 4°C for 5 min, resuspend the cell pellet with an equal volume of 50 mM potassium phosphate buffer (pH 7.0), and use an ultrasonic homogenizer (VC130PB, Sonics & Materials, Inc., Newtown, Conn. ) to break up the cells. The crushed mixture was centrifuged at 12000 rpm and 4°C for 10 minutes, and the supernatant was used for enzyme activity test. Transaminase activity was measured by the concentration of pyruvate formed from alanine and glyoxylate. Reaction system: 10mM alanine, 10mM glyoxylic acid, 1mM pyridoxal-5'-phosphate, 50mM potassium phosphate (pH7.0) and an appropriate amount of enzyme, the reaction is carried out at 30°C for 2-6 hours, and then Quantitati...
Embodiment 3
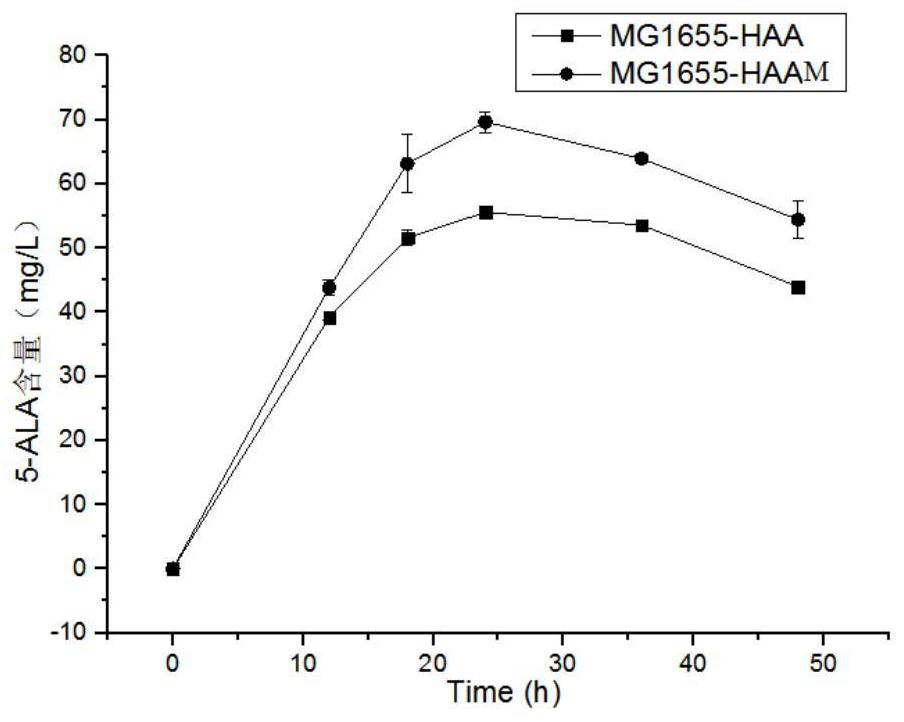
[0061] Applications of alanine-glyoxylate transaminase mutants:
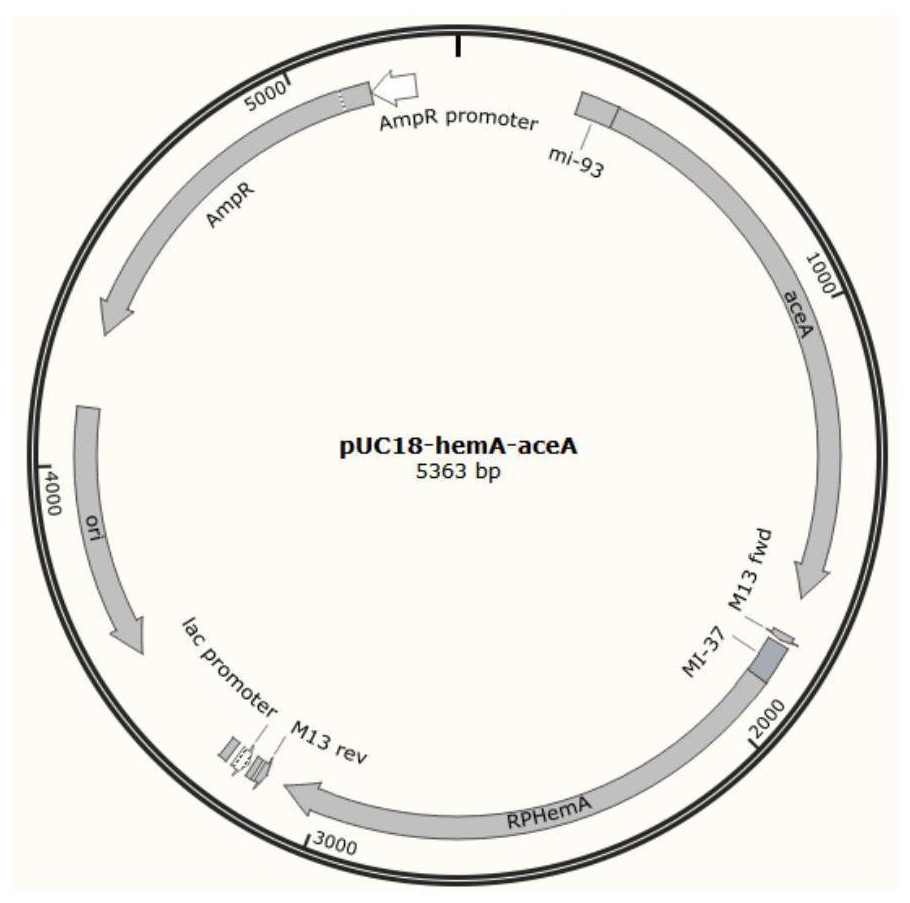
[0062] pZY48-agxT carrying the alanine-glyoxylate transaminase mutant M Its control plasmid pZY48-agxT and the pUC18-hemA-aceA plasmid shown in SEQ ID No.9 were co-transformed into Escherichia coli E.coli MG1655 respectively to obtain bacterial strain MG1655-HAAM and its control bacterial strain MG1655-HAA, and carry out 5- ALA fermentation, the specific steps are as follows:
[0063] (1) Activated bacterial strains: Streak the claim 10 carrying the alanine-glyoxylate transaminase mutant strain MG1655-HAAM and its control strain MG1655-HAA in the LB solid medium, cultivate at 37°C for 10-20h, and make strain rejuvenation;
[0064] (2) Cultivation of seed liquid: Inoculate the activated bacteria obtained in step (1) into LB liquid medium, cultivate at 37°C and 220rpm for 10-20h, and then inoculate the obtained bacterial liquid according to the initial OD 600 =0.02 inoculum amount was inoculated into fresh LB m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com