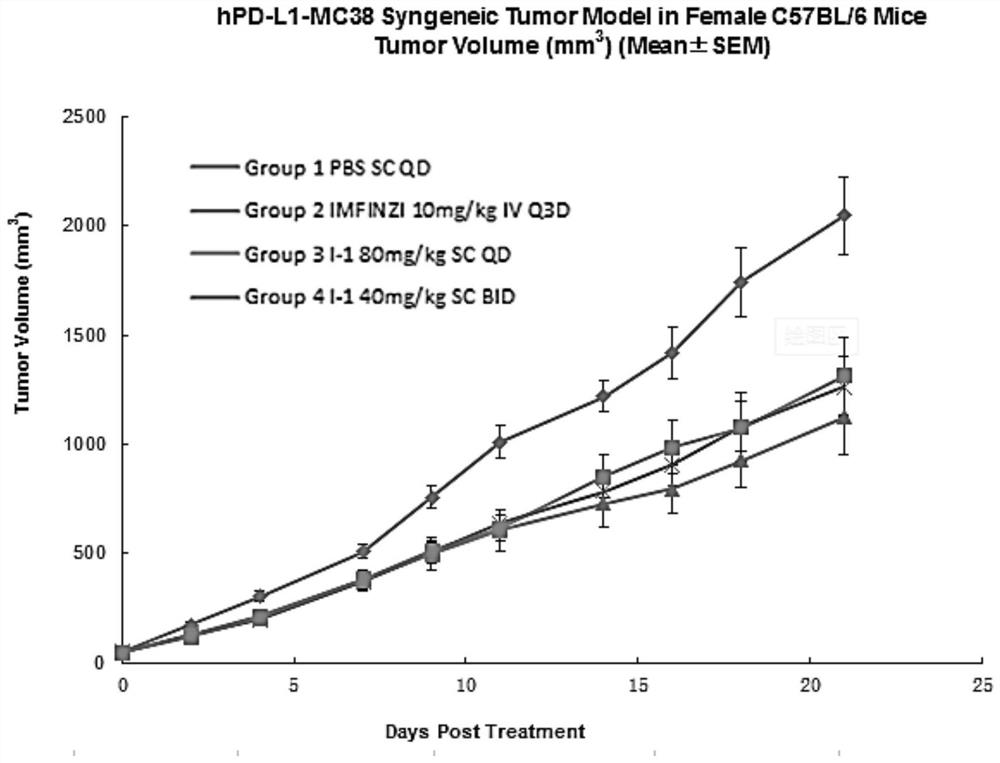
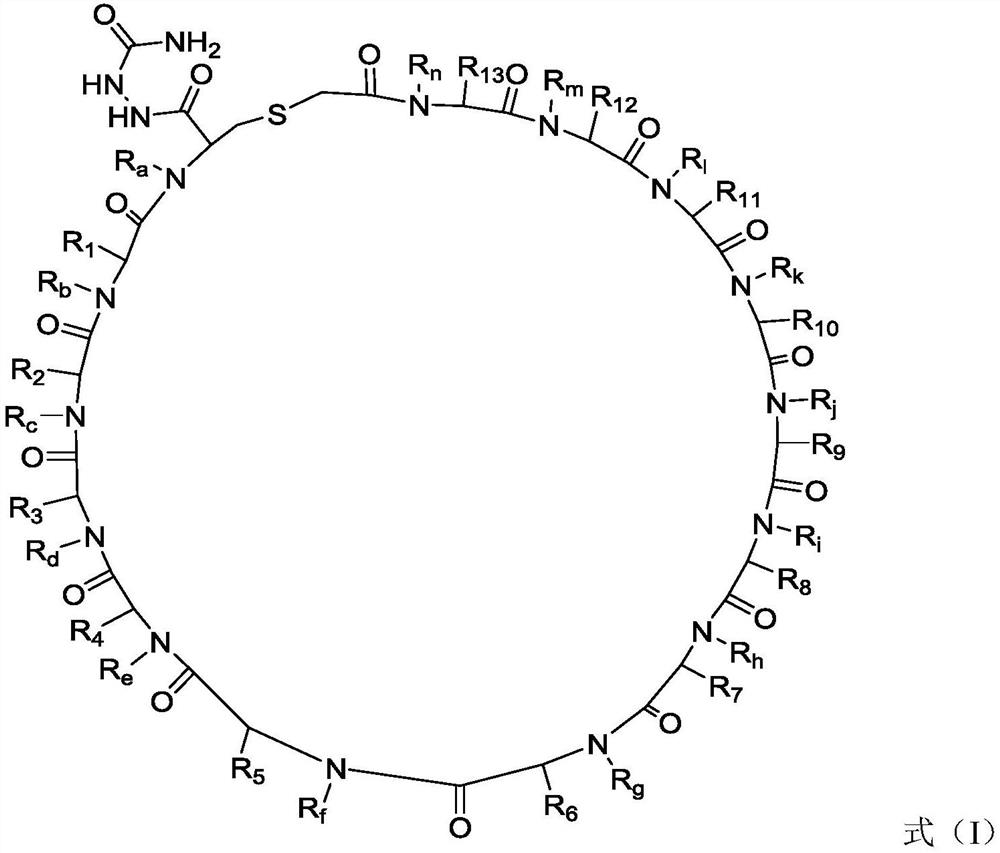
PD-L1 cyclopeptide inhibitor containing hydrazide structure
A technology of solvates and tautomers, applied in the field of cyclic peptide compounds, can solve the problem of not being able to block the interaction of PD-1, and achieve good pharmacokinetic characteristics, tumor cell growth inhibition, and a suitable half-life. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The preparation of embodiment 1 compound I-1
[0066] 1) Synthesis of fragment 1-T1 resin
[0067] Weigh 50g of Rink Amino MBHAResin with a substitution degree of 0.324mmol / g, add 500mL of DCM, bubble nitrogen, swell for 30min, and remove the solvent by suction filtration. DCM (150 mL*3) was added to wash the filter cake. Add 400mL of 20% Pip / DMF (DMF:Pip; 80:20, volume ratio) solution and mix, stir for 5 minutes, and filter cake with suction; then add 400mL of 20% Pip / DMF solution, stir for 15 minutes, and filter with suction. The filter cake was washed successively with appropriate amount of DMF and DCM and sucked dry. 200 mL of a DCM solution containing 9.84 g of phenyl p-nitrochloroformate was added to the bottle. Under the conditions of nitrogen bubbling and stirring, 12.60 g of DIEA was added dropwise, and the mixture was stirred until the ninhydrin detection method monitored the reaction to be complete. Filter and drain. The filter cake was washed successive...
Embodiment 7
[0093] Example 7. The inhibitory activity of the compound of the present invention on PD-1 / PD-L1 protein-protein interaction Evaluation of these effects shows that the compound of the present invention has a significant inhibitory effect on PD1 / PD-L1.
[0094] The specific test method is as follows:
[0095] Experiment purpose and principle
[0096] HTRF (Homogeneous Times-Resolved Fluorescence) is a technique used to detect analytes in pure liquid phase systems. This technology is based on the two major technologies of fluorescence resonance energy transfer (FRET, Fluorescence Resonance Energy Transfer) and time-resolved fluorescence (TRF, Time-Resolved Fluorescence), opening a high-throughput drug screening tool.
[0097] Experimental Materials and Instruments
[0098] The HTRF kit was purchased from Cisbio (CAT#63ADK000CPAPEG), which contains Anti-Tag1-Cyptate, Anti-Tag2-XL665 / d2, Tag1-PD-L1, Tag2-PD-1, Dilution Buffer, Detection Buffer and other reagents required for exp...
Embodiment 8
[0111] Embodiment 8. Pharmacokinetic experiment
[0112] Experimental materials for the pharmacokinetic study of compound PD-1 in SD rats given to compound I-1 by single intravenous injection or subcutaneous injection
[0113] SD rats, half male and half male, SPF grade.
[0114] Test article preparation
[0115] For intravenous injection and subcutaneous injection, use 5% DMSO + 10% Solutol + 85% sterile water for injection;
[0116] Experimental groupings Compound I-1 of the present invention was administered in groups according to the following administration methods of groups 1-5, as shown in the following table.
[0117]
[0118] Method of administration
[0119] Weigh before administration, and calculate the dosage according to body weight. It is administered intravenously and subcutaneously.
[0120] Blood collection time point
[0121] IV: Before administration, 5min, 15min, 30min, 1h, 2h, 4h, 6h, 8h, 24h after administration. PO: before administration, 15min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com