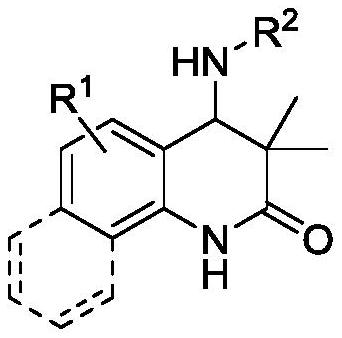
Synthetic method and anticancer activity of 4-amino dihydroquinolinone compound
A technology of aminodihydroquinolinone and synthesis method, which is applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problems of poor functional group tolerance, harsh reaction conditions, and difficult to obtain raw materials, etc., and achieve functional group tolerance Good, raw materials are easy to get, and the effect of raw materials is cheap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Into a 15mL reaction tube, sequentially add compound 2a, catalyst 1, catalyst 2, additives, Molecular sieves and an organic solvent were stirred at room temperature for 5 minutes, and then compound 1a and cesium fluoride were added sequentially, the reaction tube was sealed under air conditions, and placed in a heating module to raise the temperature and stir for reaction. After the reaction is finished, cool to room temperature, add saturated sodium bicarbonate solution to quench the reaction, filter with diatomaceous earth, and extract the filtrate with ethyl acetate. / ethyl acetate=3 / 1) afforded the product 3a as a white solid.
[0031] A series of results were obtained by changing the reaction solvent, catalyst 1, catalyst 2, additives, reaction temperature and material ratio, etc., as shown in Table 1.
[0032] Synthesis of 3a under different conditions in table 1 a
[0033]
[0034]
Embodiment 2
[0036]
[0037] Into a 15mL pressure-resistant tube, add 2a (38mg, 0.2mmol), palladium acetate (2.2mg, 0.01mmol), silver carbonate (27.6mg, 0.1mmol), Molecular sieves (40mg), 1-adamantanecarboxylic acid (36.0mg, 0.2mmol) and acetonitrile (2mL), stirred at room temperature for 5 minutes, then added 1a (59.7mg, 0.2mmol) and cesium fluoride (60.8mg, 0.4 mmol), then the reaction tube was sealed and placed in an oil bath at 100°C for 10 h. After the reaction, the reaction system was cooled to room temperature, and saturated sodium bicarbonate solution was added to quench the reaction, filtered with diatomaceous earth, the filtrate was extracted with ethyl acetate, after the organic phase was dried, suction filtered, spin-dried, and separated by a silica gel column ( Petroleum ether / ethyl acetate=3 / 1) gave the product 3a as a white solid (29.3 mg, 55%). The characterization data of this compound are: 1 H NMR (400MHz, CDCl 3 ):δ9.47(s,1H),7.24(d,J=7.6Hz,1H),7.20-7.16(m,3H),6.9...
Embodiment 3
[0039] According to the method and steps of embodiment 2 a,b , by changing reactant 1 and reactant 2, various 4-aminodihydroquinolinone compounds 3 can be synthesized, and the specific results are as follows:
[0040]
[0041]
[0042] Representative product characterization data are as follows:
[0043] 3,3-Dimethyl-4-(p-tolylamino)-3,4-dihydroquinolin-2(1H)-one(3b)
[0044] 1 H NMR (400MHz, CDCl3 ):δ8.97(s,1H),7.26-7.24(m,1H),7.21(t,J=7.6Hz,1H),7.00-6.94(m,3H),6.86(d,J=7.6Hz, 1H), 6.61(d, J=8.4Hz, 2H), 4.46(s, 1H), 3.70(br s, 1H), 2.24(s, 3H), 1.32(s, 3H), 1.21(s, 3H) . 13 C NMR (100MHz, CDCl 3 ): δ176.4, 145.6, 135.5, 130.0, 128.5, 127.3, 127.2, 126.2, 123.4, 115.3, 113.5, 59.6, 43.3, 22.9, 20.4, 19.1. HRMS (ESI) m / z: [M+Na] + Calcd for C 18 h 20 N 2 NaO 303.1468; Found 303.1459.
[0045] 4-((4-Ethylphenyl)amino)-3,3-dimethyl-3,4-dihydroquinolin-2(1H)-one(3c)
[0046] 1 H NMR (600MHz, CDCl 3 ):δ8.86(s,1H),7.26(d,J=7.8Hz,1H),7.21(td,J 1 =7.8Hz,J 2 =1.2Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com