Preparation method of pyridine derivative
A methoxypyridine and compound technology, applied in the field of pharmaceutical synthesis, can solve problems such as being unfavorable for industrial production, large amount of palladium catalyst and ligand, long reaction time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
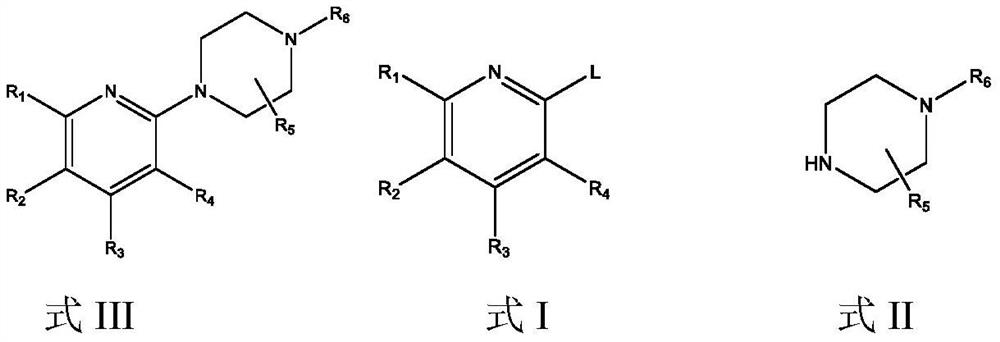
[0047] The preparation of embodiment 1 formula I compound
[0048]
[0049] Step 1: Add 101.4kg of 2,4-dichloropyridine, 305L of methanol and 142kg of potassium carbonate (powder) into the enamel reaction tank, heat up and reflux for more than 20 hours (monitored by HPLC to less than 3% of 2,6-dichloropyridine). After the reaction is completed, cool down to 10±3°C, stir and crystallize for 30 minutes, and filter. The temperature of the filtrate was controlled below 35°C, and 40kg of HCl gas was slowly introduced. After passing through, stir the reaction for more than 30 minutes, and concentrate under reduced pressure at 30-35°C. Concentrate until no obvious liquid flows out, add 60L ethyl acetate and continue to concentrate for 1.5h. After concentration, add 345L of ethyl acetate, stir for 30min, cool down to 0-5°C and continue to stir for 2.5h, shake off and dry to obtain the hydrochloride of the compound of formula Ⅰ-1 with a wet weight of 112.24kg, vacuum at 40-50°C D...
Embodiment 2
[0062]
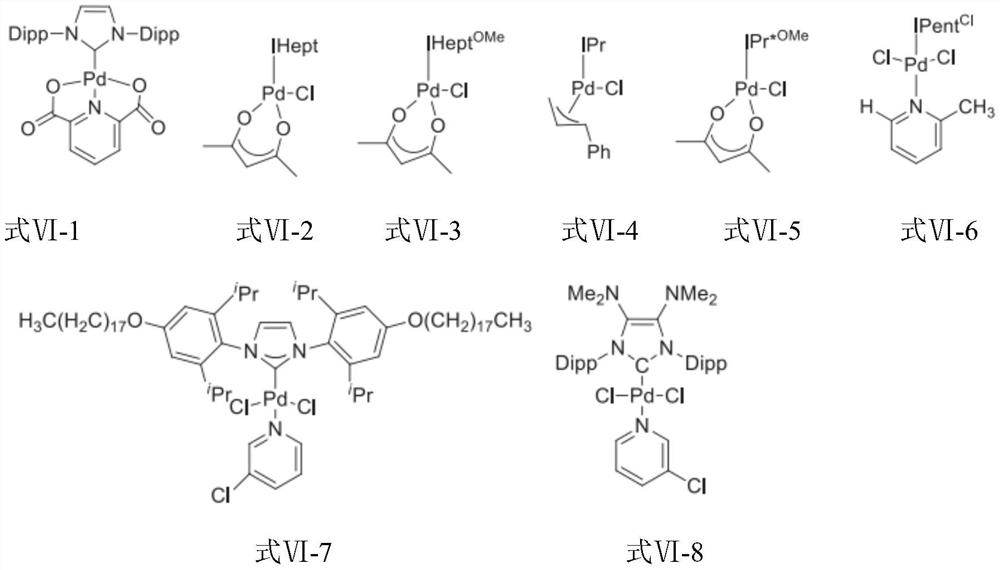
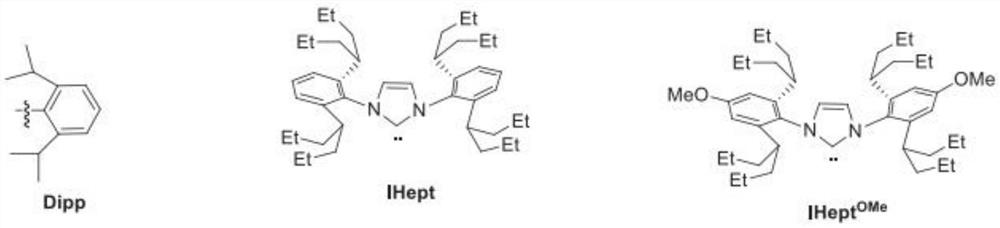
[0063] Step 1: Add 1400.9g of 2-chloro-4-methoxypyridine, 1691.8g of (S)-3-methyl-1-tert-butoxycarbonylpiperazine, 16L of dry toluene and tert-butanol to the reactor Potassium 1904.9g, after stirring evenly, add Pd 2 (dba) 3 155.5g, BINAP212.2g, nitrogen replacement 3 times, under nitrogen atmosphere, reflux for 10h, TLC showed that the reaction of raw materials was complete. Add 10L of purified water, separate the layers, extract the aqueous layer twice with ethyl acetate 10L*2, combine the organic layers, wash twice with 20L of saturated brine, dry over anhydrous sodium sulfate, filter, and evaporate the filtrate under reduced pressure until viscous. used directly in the next reaction.
[0064] Step 2: In the reaction kettle, add (S)-4-(4-methoxypyridin-2-yl)-3-methylpiperazine-1-carboxylic acid tert-butyl ester in acetonitrile solution, cool down to 0°C 1290.9 g of bromosuccinimide was added in batches with stirring. After adding, warm up to room temperature a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com