Method for synthesizing salicylamide by continuous method
A salicylamide, continuous process technology, applied in the field of synthesizing salicylamide, can solve the problems of high production cost, high cost, difficulty in large-scale industrialization, and high energy consumption, save energy and cost for purification, realize continuous reaction, product high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-29
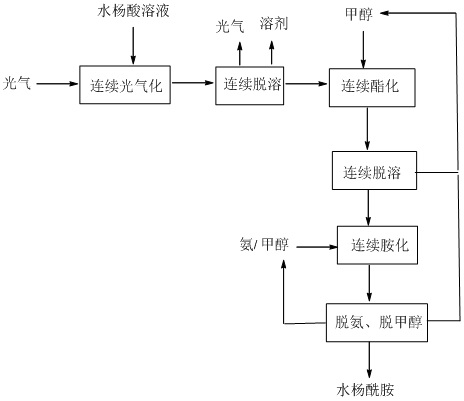
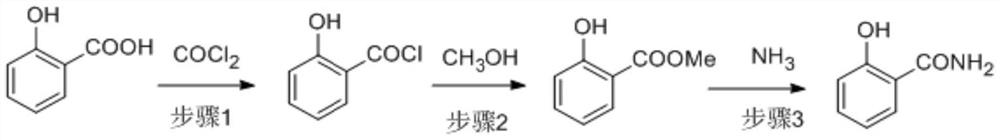
[0039] The salicylic acid solution (1 times equivalent) and phosgene are continuously entered into the acyl chloride reactor in a certain ratio to react to generate salicyloyl chloride, and the obtained salicyloyl chloride solution enters a continuous precipitation device to remove excess phosgene and solvent. Enter into the esterification reactor with methanol in a certain proportion for continuous reaction to generate methyl salicylate, and the obtained methyl salicylate solution enters the continuous desolventizing equipment to remove hydrogen chloride gas and excess methanol, and the obtained methyl salicylate The ammonia solution and the methanol solution of ammonia enter the continuous ammoniation reactor to synthesize salicylamide. The salicylamide solution finally undergoes continuous deamination and demethanol to obtain salicylamide. The removed ammonia and methanol return to ammoniation and ester after recovery. Chemical section.
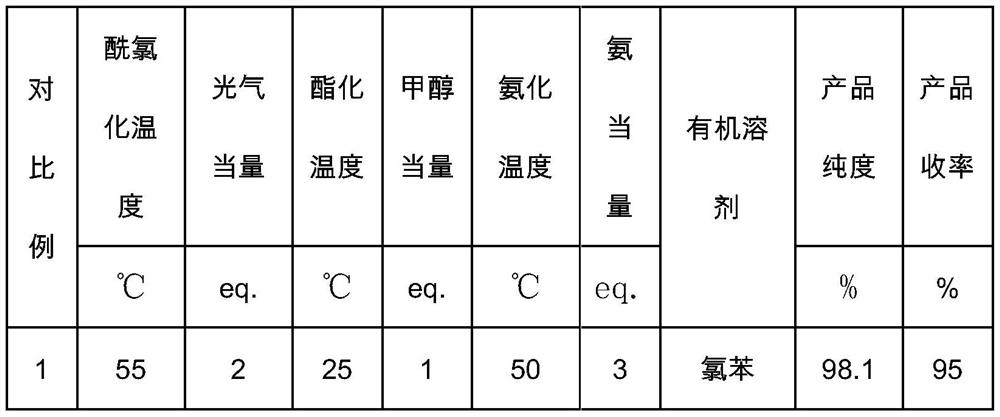
[0040] The reaction parameters and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com