Pharmaceutical composition containing dozagliptin and sitagliptin and preparation method thereof
A composition and compound technology, applied in the pharmaceutical composition containing dozagliliptin and sitagliptin and its preparation, improving the field of drug combination for type II diabetes, capable of solving the problem of metformin's large toxic and side effects and reducing drug effects Insufficient effect and long-term effect of drugs, etc., to avoid toxic and side effects, improve β-cell function, and improve drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 A preparation method of pharmaceutical composition X1 containing dozagliliptin and sitagliptin
[0033] The present embodiment provides a preparation method of pharmaceutical composition X1 containing dozagliliptin and sitagliptin, which comprises the following steps:
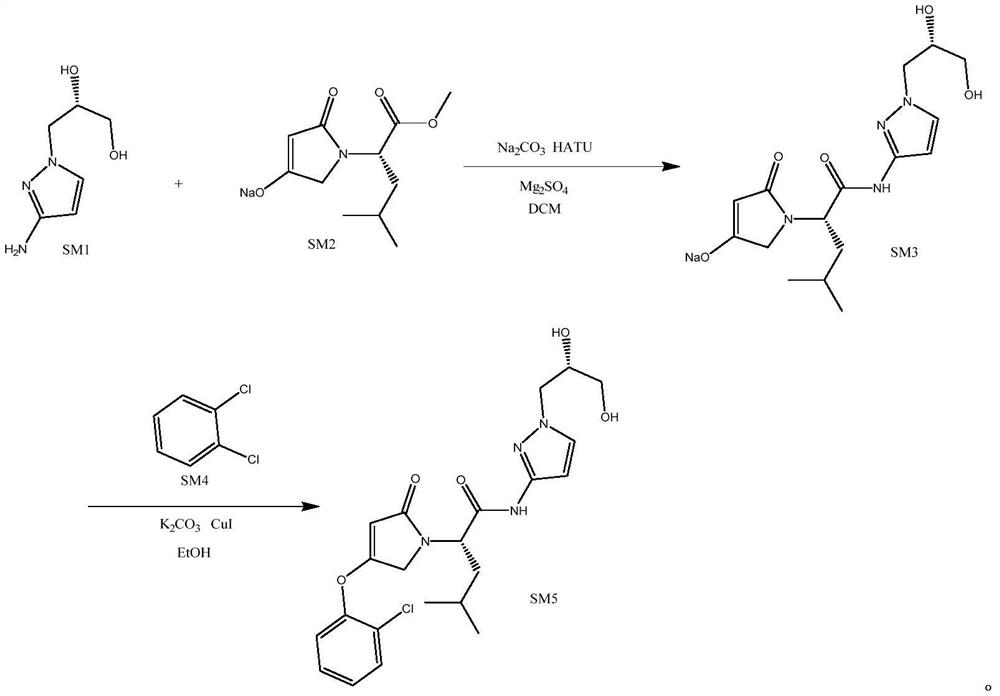
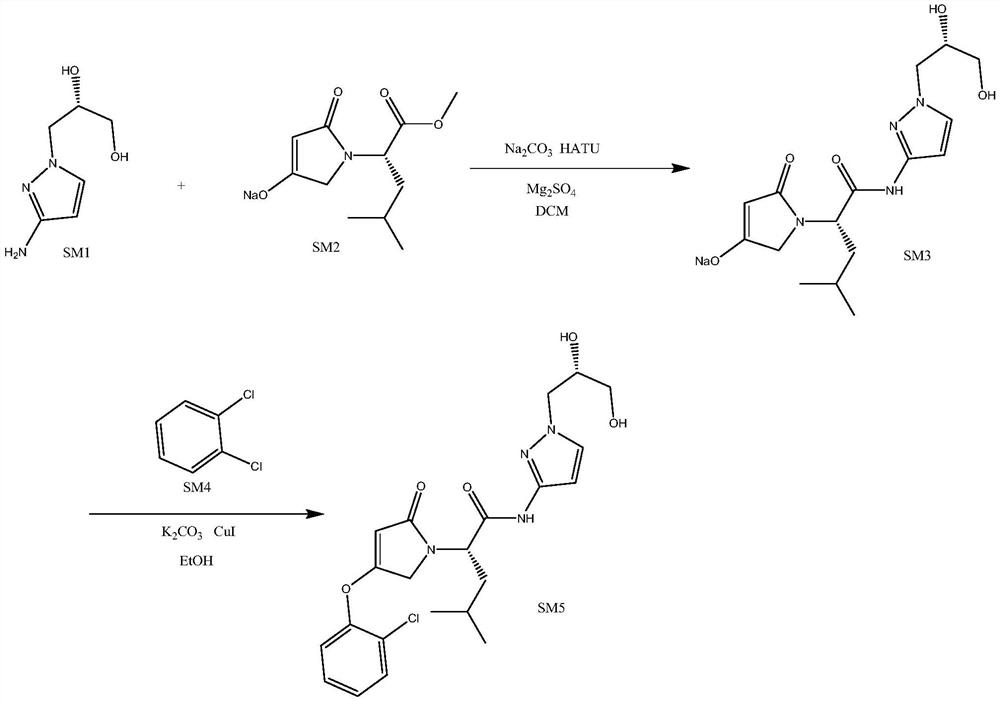
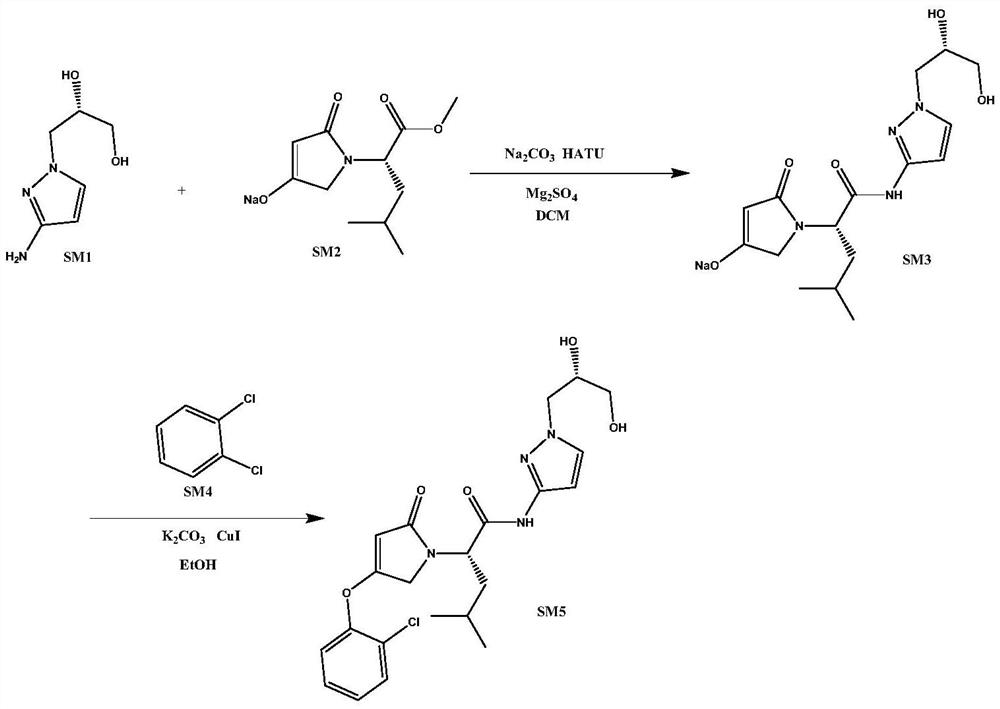
[0034] S1. Synthesis of dozaglietin
[0035] T1, take by weighing the compound shown in 157g formula SM1 and the compound shown in 249g formula SM2 respectively and join in 500ml dichloromethane and mix uniformly, add 100g anhydrous magnesium sulfate, stir 5min, add 570g HATU (1.5eq) and 318g sodium carbonate ( 3eq), after mixing evenly, heat to 40°C and keep warm for reaction, react for 4h, use TLC to monitor the progress of the reaction during the reaction, until the fluorescent spots of the compounds shown in formula SM1 and formula SM2 on the silica gel plate disappear, that is, the end of the reaction, the reaction After finishing, filter and rinse filter cake with 300ml ethyl acetate, co...
Embodiment 2~6
[0042] Examples 2-6 Preparation methods of pharmaceutical compositions X2-X6 containing dozagliliptin and sitagliptin
[0043] The present Examples 2-6 provide the preparation method of pharmaceutical compositions X2-X6 containing dozagliliptin and sitagliptin, the preparation method is basically the same as that of Example 1, the only difference is that some process parameters are different, the specific process The parameters are shown in Table 1.
[0044] Table 1: Preparation process table of pharmaceutical compositions X2-X6 containing dozaglietin and sitagliptin
[0045]
[0046] Other processing parameters are all identical with embodiment 1.
experiment example 1
[0047] Experimental example 1 repeated administration test
[0048] Randomly select 18 mice with similar physical signs, good health and half male and half male for repeated administration test, randomly divide 18 half male and half male mice into three groups, the first group is fed with a single sitagliptin Hypoglycemic drugs, the second group fed the pharmaceutical composition of sitagliptin and metformin, and the third group fed the pharmaceutical composition X3 containing dozaglietin and sitagliptin prepared in Example 3;
[0049] Three groups of experiments were administered orally. At the beginning of the test, the dosage was administered at a daily rate of 2 mg / kg. After 3 weeks, the dosage was increased, and the dosage was changed to 10 mg / kg. After feeding again for 3 weeks, the improvement The dosage of the drug is 50mg / kg, and the feeding is continued for 3 weeks. The dosage of no adverse reactions in the test is 10mg / kg. Calculated according to the daily recommend...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com