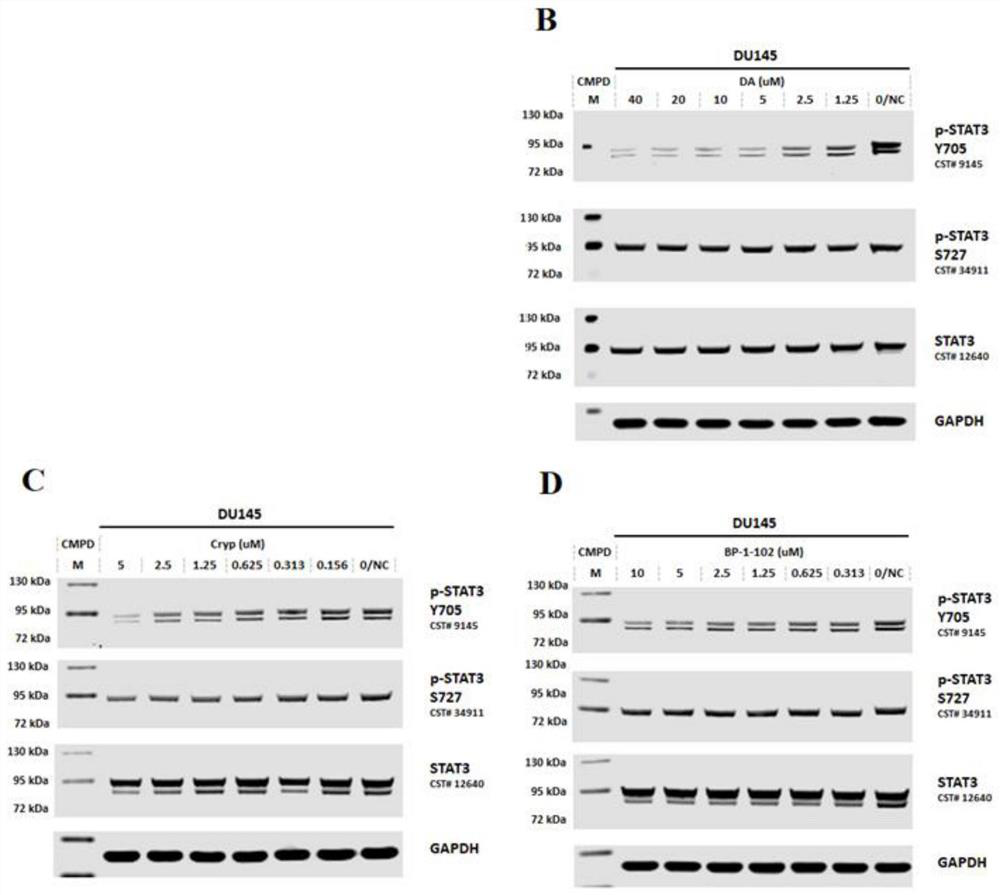
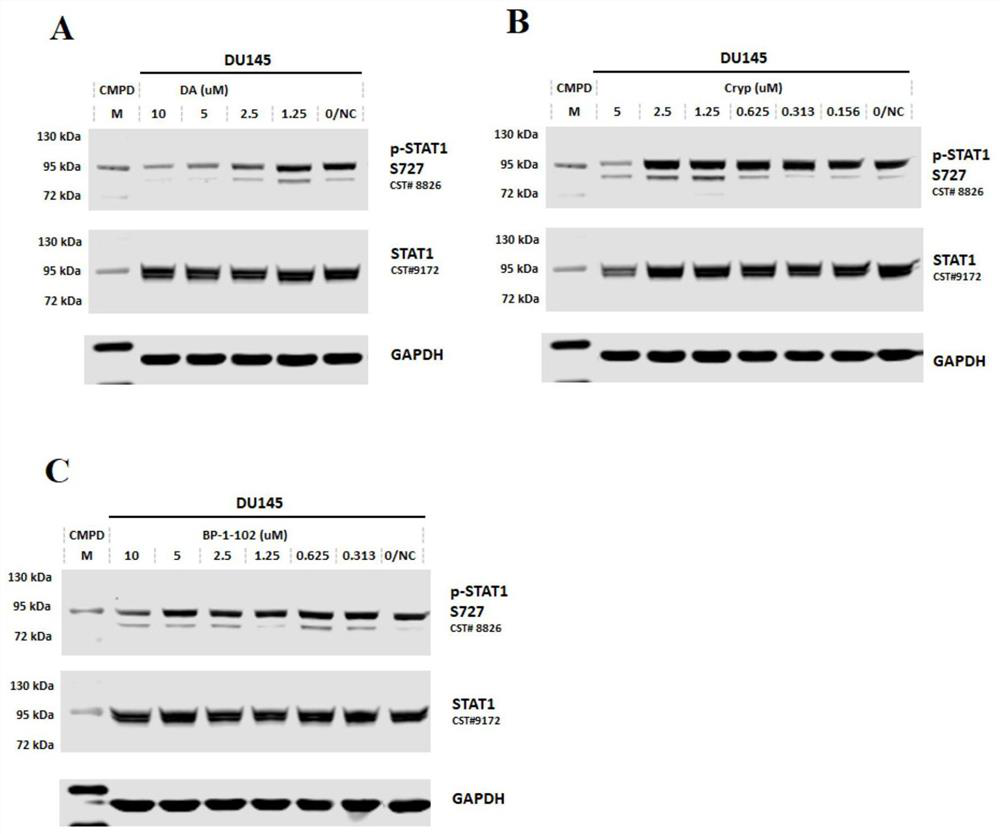
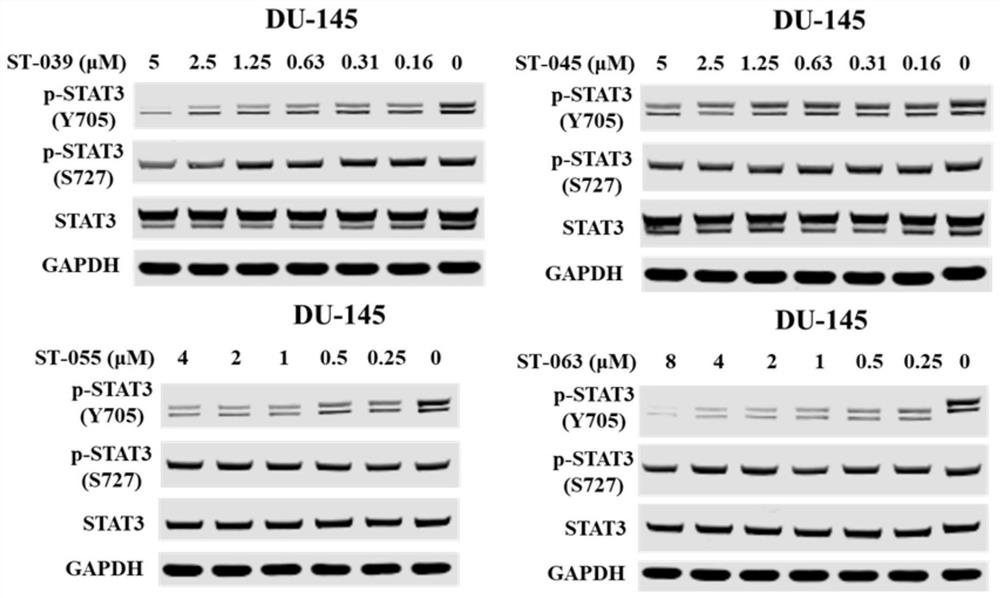
Heteroaromatic amide compound and preparation method and application thereof
A technology of heteroarylamides and compounds, which is applied in the field of heteroarylamide compounds, can solve the problem of single STAT3 inhibitors, etc., and achieve the effect of strong inhibition of tumor cell proliferation activity, high selectivity, and good market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241] Synthesis of Example 1 Compound 2-(4-(2-formyl-3-methylcyclopent-1-en-1-yl)benzamido)thiophene-3-carboxamide (ST-013)
[0242] Preparation of Intermediates Synthesis of Compound 5-methyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)cyclopent-1-ene-1-carbaldehyde(3)
[0243]
[0244] Measure DMF (2.37mL, 30.6mmol) and dissolve it in anhydrous dichloromethane (35mL). When the ice bath drops to 0°C, add PBr dropwise 3 (2.42mL, 25.5mmol), kept stirring at 0°C for 1 hour. Subsequently, compound 1 (1.0 g, 10.2 mmol) was dissolved in anhydrous dichloromethane (15 mL), and slowly added dropwise to the above reaction solution. After the reaction was dropped, it was slowly raised to room temperature and stirred for 12 hours. When the reaction solution was cooled to 0 °C, water (50 mL) was added to the reaction solution to quench, and the reaction solution was dissolved with NaHCO 3 The solid neutralized the solution, extracted with dichloromethane (3×50 mL), and the organi...
Embodiment 2
[0251] Synthesis of Example 2 Compound (R)-2-(4-(2-formyl-3-methylcyclopent-1-en-1-yl)benzamido)thiophene-3-carboxam-ide (ST-022)
[0252] Synthesis of compound 3a
[0253]
[0254] Compound 3a was synthesized the same as compound 3, and the purchased starting materials were replaced with 1a.
[0255]
[0256] Weigh compound 3a (100mg, 0.53mmol), compound 50 (0.26mmol), Pd(dppf)Cl 2 (19mg, 0.026mmol) and anhydrous sodium carbonate (55mg, 0.52mmol) were dissolved in tetrahydrofuran (10mL) and water (1mL). Under the protection of argon, the temperature of the reaction solution was raised to 75°C and stirred for 1 hour. The above reaction solution was concentrated to obtain a crude product, which was purified by flash column chromatography (PE / EtOAc=2 / 1) to obtain ST-022 (21 mg, 23%) as a yellow solid.
[0257] 1 HNMR (500MHz, Chloroform-d) δ12.98(s,1H),9.82(s,1H),8.06(d,J=7.8Hz,2H),7.49(d,J=7.8Hz,2H),7.13– 6.98(m,1H),6.87(s,1H),5.86(d,J=106.6Hz,2H),3.19–2.95(m,2H),2.74...
Embodiment 3
[0258] Synthesis of Example 3 Compound 2-(4-(pyrimidin-5-yl)benzamido)thiophene-3-carboxamide (ST-024)
[0259]
[0260] Weigh compound 89 (66mg, 0.53mmol), compound 50 (84mg, 0.26mmol), Pd(dppf)Cl 2 (19mg, 0.026mmol) and anhydrous sodium carbonate (55mg, 0.52mmol) were dissolved in tetrahydrofuran (10mL) and water (1mL), and the reaction solution was heated to 85°C under the protection of argon, and stirred for 1 hour. After the reaction solution was concentrated to dryness, it was purified by flash column chromatography (PE / EtOAc=1 / 3) to obtain ST-024 (30 mg, 36%) as a yellow solid. 1 HNMR (500MHz, DMSO-d 6 )δ9.24(s,3H),8.13(s,1H),8.06(q,J=8.1Hz,4H),7.67(s,1H),7.57–7.49(m,1H),7.05(d,J =5.8Hz,1H); 13 CNMR (125MHz, DMSO-d 6 )δ167.82,162.34,158.34,155.51,146.51,138.26,132.70,132.55,128.31,128.21,123.76,117.07,116.28; HRMS(ESI):m / z calcd for C 16 h13 N 4 o 2 S[M+H] + :325.0754, found 325.0750.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com