Method for accurately testing fuel cell anode overpotential caused by hydrogen impurities and application thereof
A fuel cell and overpotential technology, applied in fuel cells, measuring current/voltage, measuring electrical variables, etc., can solve the problems of inaccurate determination of anode overpotential and difficult polarization, and achieve the effect of simple and easy test method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
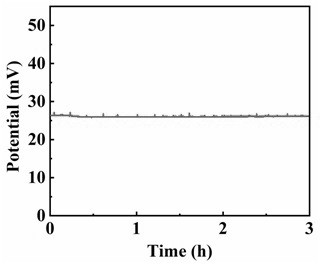
[0023] Make the active area 25 cm 2 The membrane electrode was assembled into the single cell fixture and connected to the test instrument. After checking the airtightness of the fixture, the working temperature was set at 75 °C, and pure hydrogen was fed to the anode. The yield was 95%, the cathode was not ventilated, and the gas pressure for atmospheric pressure. After the temperature rises to 75 ℃, the constant current operation is stable ( figure 1 ), the voltage stabilized at about 26 mV.
Embodiment 2
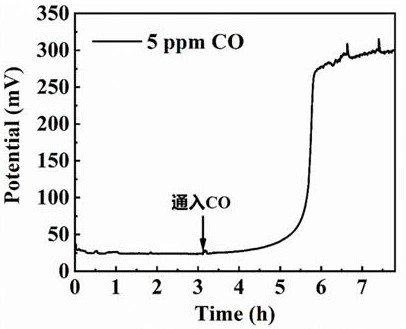
[0025] The operating conditions are the same as in Example 1, except that the pure hydrogen fed into the anode side in step (1) is mixed with 5 ppm CO. Constant current running display ( figure 2 ), the overpotential rises by about 270 mV after the impurities are poisoned.
Embodiment 3
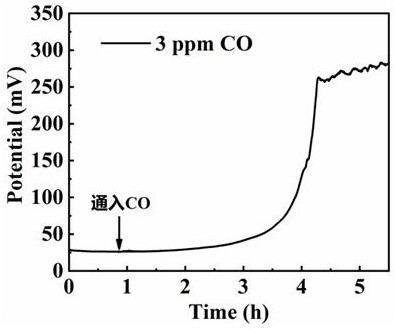
[0027] The operating conditions are the same as in Example 1, except that the pure hydrogen fed into the anode side in step (1) is mixed with 3 ppm CO, and the temperature is 65 °C. Constant current running display ( image 3 ), after the impurities are poisoned, the overpotential rises by about 250 mV.
[0028] It can be seen that the overpotential generated when the impurities on the anode side pass through the electrochemical hydrogen pump can effectively avoid the polarization effect caused by the redox reaction of the cathode in the fuel cell.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com