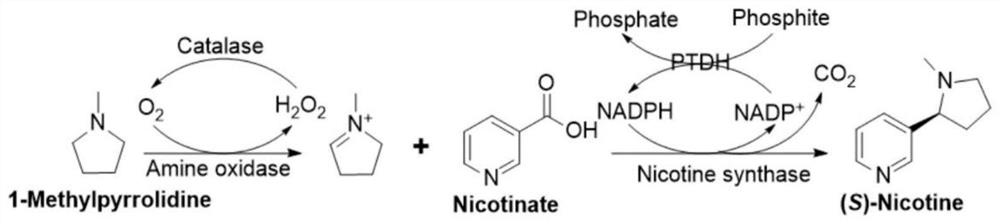
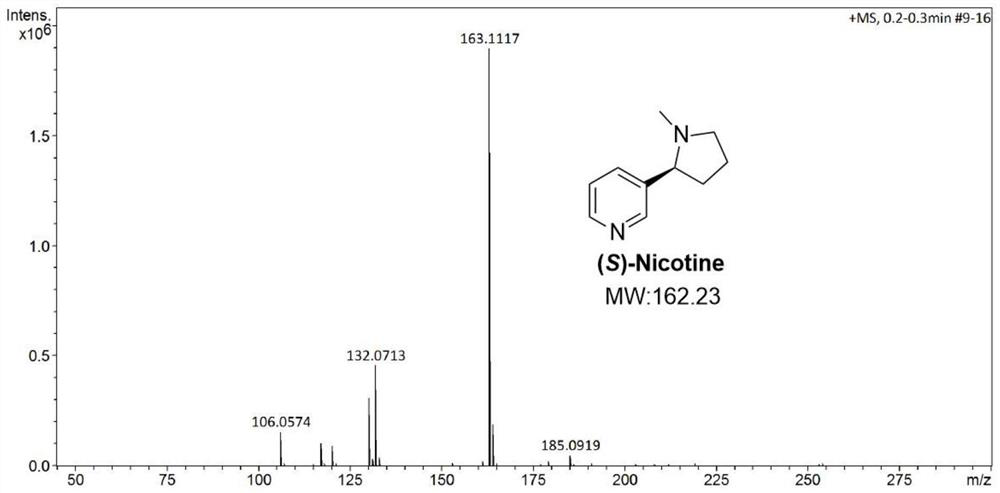
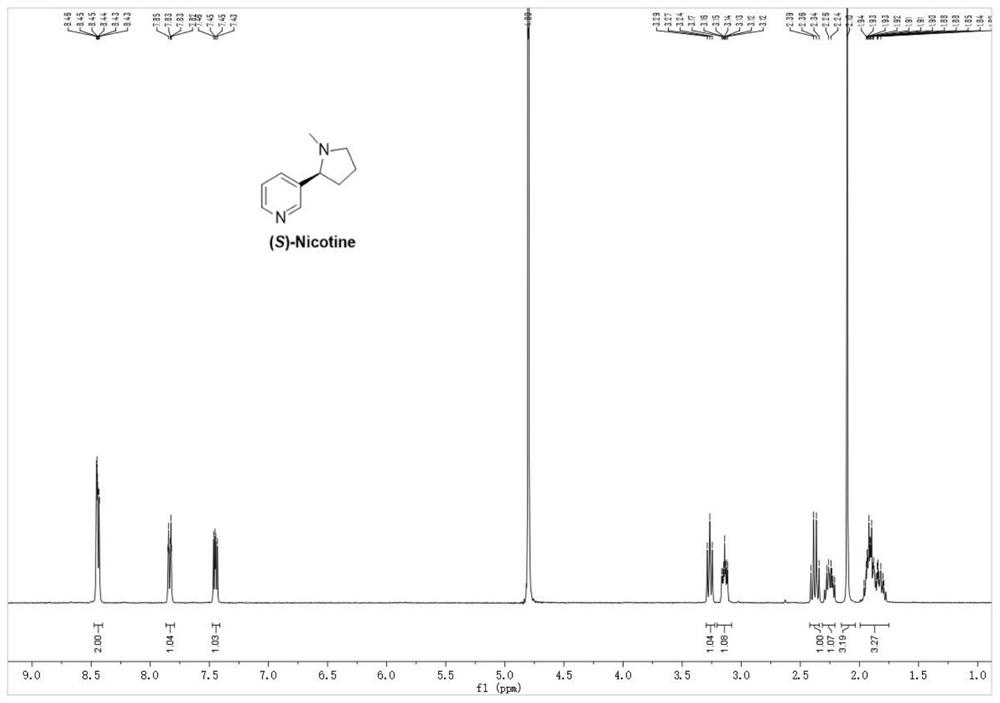
Preparation method of S-nicotine
A nicotine and amino acid technology, applied in the field of S-nicotine preparation, can solve the problems of high environmental and safety costs, expensive chemical catalysts, and short overall routes, and achieve low production costs, low prices, and wide sources of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] The invention provides a preparation method of S-nicotine. Those skilled in the art can refer to the content of this article to appropriately improve the process parameters to achieve. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and relevant personnel can obviously make changes or appropriate changes and combinations to the method and application herein without departing from the content, spirit and scope of the present invention to realize and apply the present invention Invent technology.
[0079] Unless otherwise specified, the test materials used in the present invention are all common commercial products, which can be purchased in the market.
[0080] In the present invention, catalase (catalase) was purchased ...
Embodiment 1
[0085] Example 1 One-pot method for preparing S-nicotine by liquid enzyme (AO1, NS)
[0086] Add 17 g of 1-methylpyrrolidine (200 mM), 24.6 g of nicotinic acid (200 mM), and 3.0 g of β-nicotinamide adenine into 1 L of 50 mM pH 8.0 tris-hydrochloride (Tris.HCl) solution dinucleotide phosphate (NADP +) monosodium salt (0.4mM), 52 grams of sodium phosphite pentahydrate (240mM) and 100ml of isopropanol (substrate co-solvent). Utilize NaOH aqueous solution to adjust the pH value of the reaction solution to 8.0, add the compound enzyme to obtain the reaction solution; wherein, the compound enzyme is composed of: 2000UAO1 (SEQ ID NO:1), 4000UNS (SEQ ID NO:5), 1000U Catalase, 6000U PTDH ( SEQ ID NO: 7);
[0087] Subsequently, the reaction solution was transferred to a pressure-resistant reactor to maintain an oxygen pressure of 1.5 atmospheres at 30° C. and slowly stirred for 6 hours. After the reaction, the pH of the solution was adjusted to 10 and extracted three times with 700 ml...
Embodiment 2
[0088] Embodiment 2: liquid enzyme (AO2, NS) prepares S-nicotine in one pot
[0089] The difference from Example 1 is that the amino oxidase AO2 replaces AO1, and the other processes are the same. Similarly, 17 grams of 1-methylpyrrolidine (200 mM), 24.6 grams of nicotinic acid (200 mM), and 3.0 grams of β-nicotinamide gland Purine dinucleotide phosphate (NADP + ) monosodium salt (0.4mM), 52 grams of sodium phosphite pentahydrate (240mM) and 100ml of isopropanol. Utilize NaOH aqueous solution to adjust the pH value of the reaction solution to 8.0, add the compound enzyme to obtain the reaction solution; wherein, the compound enzyme is composed of: 2000UAO (SEQ ID NO:2), 4000UNS (SEQ ID NO:5), 1000U Catalase, 6000UPTDH ( SEQ ID NO: 7);
[0090] Subsequently, the reaction solution was transferred to a pressure-resistant reactor to maintain 1.5 atmospheric pressure of oxygen at 30° C. and stirred for 4 hours. After the HPLC detection reaction was completed and the pH of the so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com