Application of quinoline derivative in preparation of medicine for treating diabetes and complications thereof
A technology of derivatives and quinolines, which is applied in the field of application of quinoline derivatives in the preparation of drugs for the treatment of diabetes and its complications, can solve the problems of low cure rate of diabetic complications, achieve the purpose of enhancing kidney function, inhibiting cell Death, the effect of improving renal function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
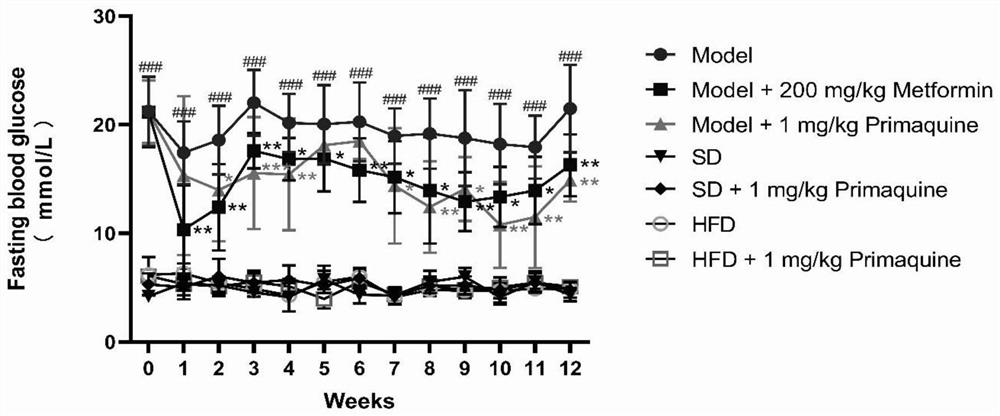
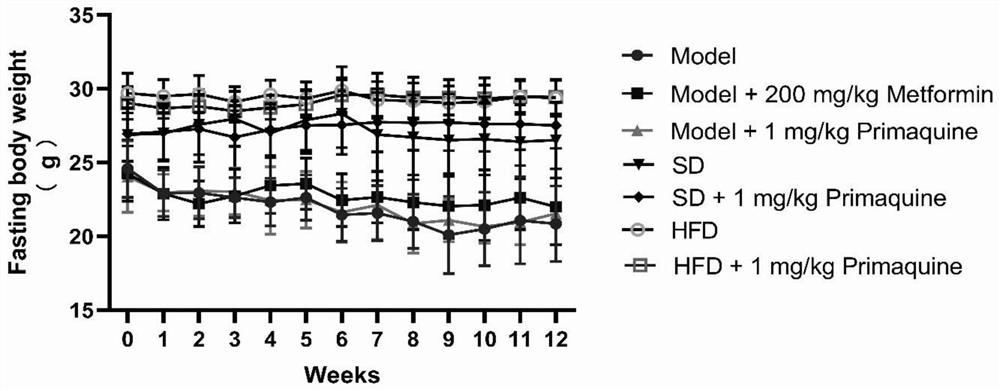
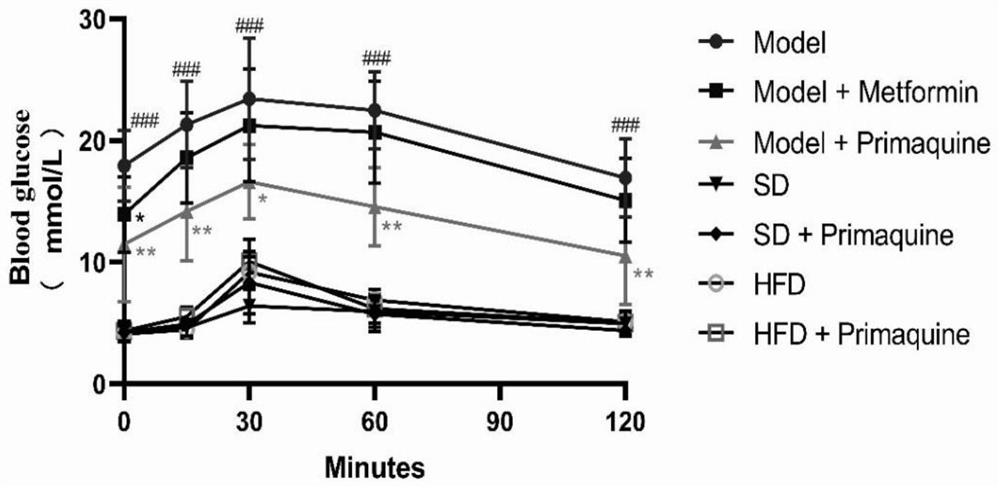
[0063] The impact of embodiment 1 primaquine phosphate on diabetes
[0064] 1. Establishment and grouping of C57BL / 6N mouse diabetes model
[0065] 1.1 Model establishment
[0066] Seventy C57BL / 6N mice were randomly divided into three groups, namely, normal diet group (20 rats), high-fat diet group (20 rats), and high-fat diet+STZ group (30 rats).
[0067] Mice in the normal feed group: After being fed with the normal feed for three weeks, they were continuously injected with an equal volume of citric acid buffer solution for 5 days, and then continued to be fed with the normal feed until the modeling was completed;
[0068] Mice in the high-fat diet group: After being fed with high-fat diet for three weeks, they were continuously injected with an equal volume of citric acid buffer solution for 5 days, and then continued to be fed with high-fat diet until the end of modeling;
[0069] Mice in the high-fat diet+STZ group: After being fed with high-fat diet for three weeks, i...
Embodiment 2
[0105] The effect of embodiment 2 quinine on diabetic mice
[0106] 1. Establishment and grouping of C57BL / 6N mouse diabetes model
[0107] 1.1 Model establishment
[0108] The 30 mice were randomly divided into two groups: normal diet group (6 rats), high-fat diet+STZ group (24 rats); other operations were the same as in Example 1.
[0109] 1.2 Grouping
[0110] When the fasting blood glucose of the mice in the high-fat diet+STZ group (without food and water for 12 hours, blood glucose was measured at 7:30pm-9:00pm) was higher than 15mmol / L, it could be considered as a successful type 2 diabetes model. After the experiment officially started, all mice were fed with normal feed. Wherein high-fat diet+STZ group mouse model mice were divided into three groups (model group, model+metformin hydrochloride group and model+quinine group), common diet group mice were divided into one group (common diet group), a total of 4 Groups, 6 in each group, as follows:
[0111] a. Model gr...
Embodiment 3
[0122] The effect of embodiment 3 quinacrine on diabetic mice
[0123] 1. Establishment and grouping of C57BL / 6N mouse diabetes model
[0124] 1.1 Model establishment
[0125] The 30 mice were randomly divided into two groups: normal diet group (6 rats), high-fat diet+STZ group (24 rats); other operations were the same as in Example 1.
[0126] 1.2 Grouping
[0127] When the fasting blood glucose of the mice in the high-fat diet+STZ group (without food and water for 12 hours, blood glucose was measured at 7:30pm-9:00pm) was higher than 15mmol / L, it could be considered as a successful type 2 diabetes model. After the experiment officially started, all mice were fed with normal feed. Among them, the high-fat diet+STZ group mouse model mice were divided into three groups (model group, model+metformin hydrochloride group and model+quinacrine dihydrochloride group), and the mice in the common diet group were divided into one group (common diet group), a total of 4 groups with 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com