Pharmaceutical combinations comprising mebendazole and strong or moderate cyp1a2 inhibitor
A CYP1A2, mebendazole technology, applied in the field of drug combinations comprising mebendazole and strong or moderate CYP1A2 inhibitors, can solve the problems of poor absorption, insufficient bioavailability, low systemic bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0167] Certain embodiments of the invention are described in more detail below. Unless otherwise specified, the embodiments described below apply to each of the first to ninth aspects of the present invention.
[0168] The pharmaceutical composition comprises mebendazole or a pharmaceutically acceptable salt, solvate, hydrate, N-oxide, prodrug or active metabolite thereof and a strong or moderate CYP1A2 inhibitor.
[0169] In some embodiments:
[0170] - Strong or moderate CYP1A2 inhibitors are furofylline, thiabendazole, fluvoxamine, 8-phenylphylline, ciprofloxacin, enoxacin, or zafirlukast, or their pharmaceutically acceptable Salts, solvates, hydrates, N-oxides, prodrugs or active metabolites of .
[0171] In some embodiments:
[0172] - Mebendazole has an IC of 1 μM or less against neuroblastoma, sarcoma, renal or ovarian cancer derived cell lines in cell viability assays 50 ;and
[0173] - A strong or moderate CYP1A2 inhibitor is furofylline or a pharmaceutically acc...
Embodiment 1
[0204] Example 1: Investigation of whether mebendazole is a substrate of the human efflux transporters P-gp and BCRP in polarized Caco-2 cell monolayers
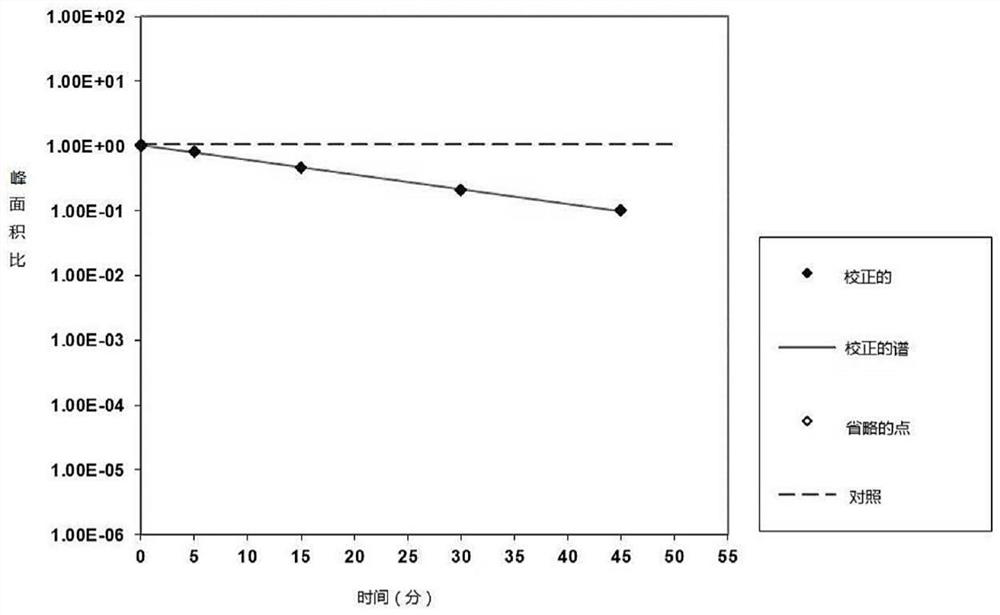
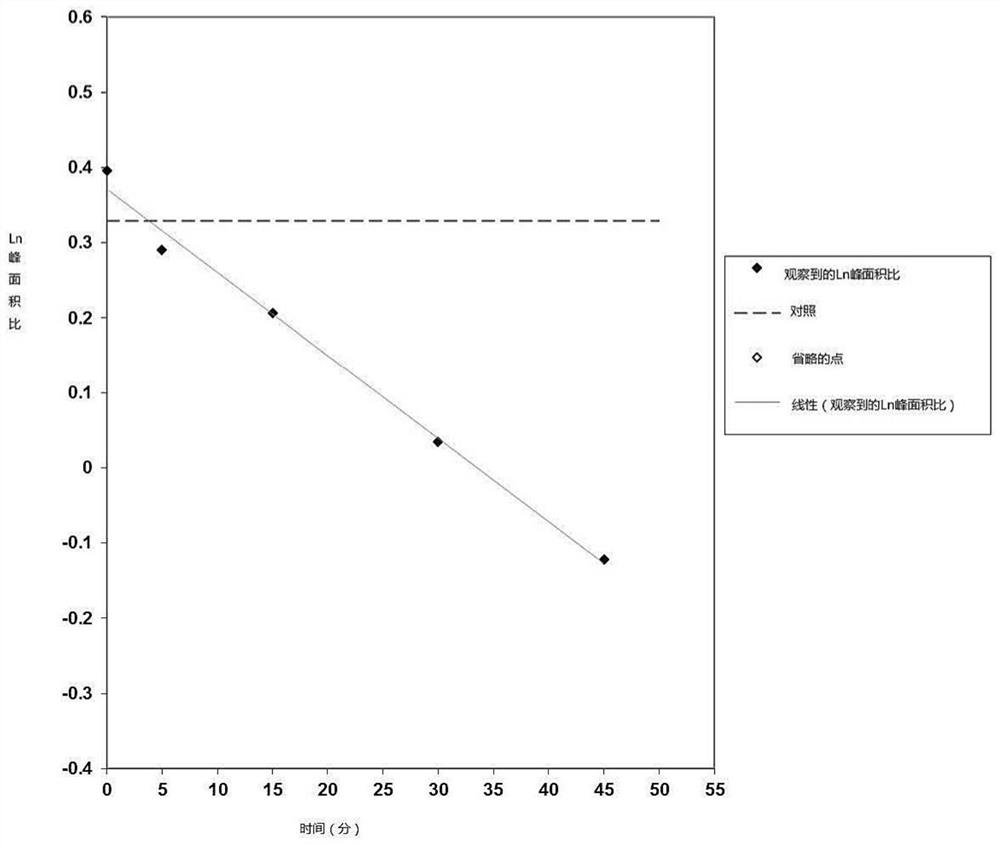
[0205] The aim of this study was to evaluate the potential of mebendazole as a substrate of the human efflux transporters P-gp and BCRP in polarized Caco-2 cell monolayers. Test compounds (10 μΜ in the absence and presence of reference inhibitors) were incubated with polarized cell monolayers for 120 min. Bidirectional apparent permeability of test compounds was quantified by LC-MS / MS and used to determine efflux ratio.
[0206] The efflux ratio of the positive control substrate in the absence and presence of the reference inhibitor was determined from incubations run with the test compound and demonstrated that the in vitro test system was able to detect the transported substrate. For the P-gp substrate assessment, the efflux ratios of tarilolol obtained in the absence (ER=52.2) and presence (ER=1.48) of the reference inhi...
Embodiment 2
[0217] Example 2: Investigation of whether mebendazole is a substrate of human SLC transporters OATP1B1 and OATP1B3 in transiently transfected HEK293 cells
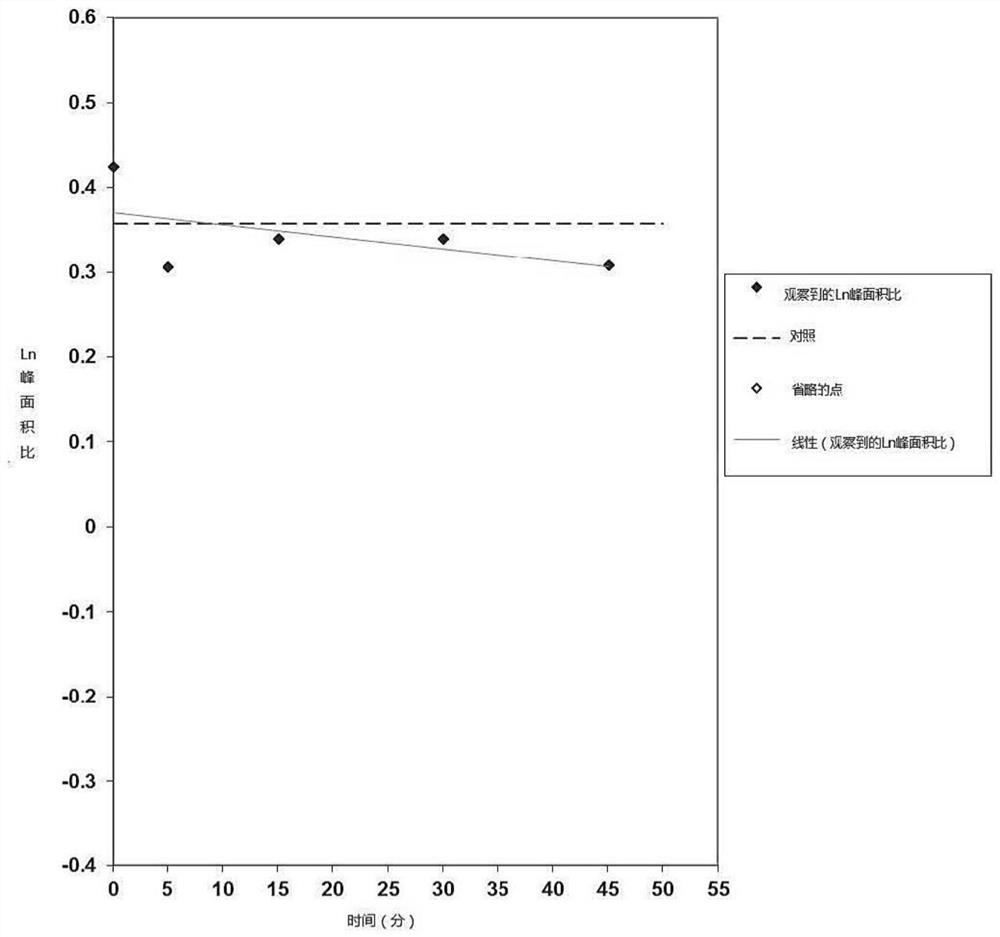
[0218] The aim of this study was to evaluate the potential of mebendazole as a substrate of human SLC transporters (OATP1B1 and OATP1B3) in transfected HEK293 cells. Test compounds (1 and 10 μΜ) were incubated with HEK293 cells expressing SLC transporter and control (empty vector) HEK293 cells for 2 min. Absorption of test compounds was quantified by LC-MS / MS and used to determine absorbance ratio.
[0219] The uptake ratio of the positive control substrate was determined from incubations run with the test compound and demonstrated that the in vitro test system was able to detect the transported substrate. The absorbance ratios obtained for [3H]-estradiol 17β-D-glucuronide confirmed that the in vitro test system is capable of detecting substrates of OATP1B1. The calculated absorbance ratios of 1 μM and 10 μM mebendazole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com