Preparation method of terephthalic acid-based ionic liquid catalyst and application of terephthalic acid-based ionic liquid catalyst in PET degradation
A terephthalic acid, ionic liquid technology, applied in chemical instruments and methods, ester group and hydroxyl reaction preparation, physical/chemical process catalysts, etc., can solve the problems of inability to achieve multiple recycling, poor stability, etc. The introduction of impurities, the improvement of purity, and the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
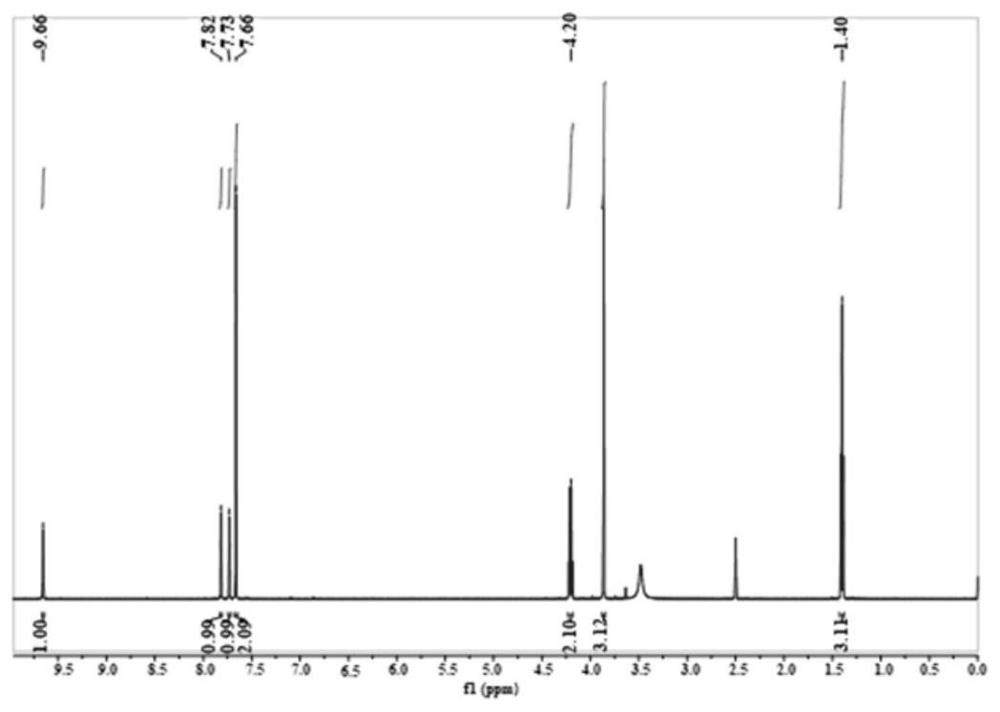
[0031] This example illustrates that 1-ethyl-3-methylimidazole terephthalate ([Emim] 2 TPA) preparation.
[0032] First prepare the 1-ethyl-3-methylimidazolium chloride salt ([Emim]Cl) aqueous solution of 1mol / L, then this aqueous solution is slowly passed through the ion-exchange column that anion-exchange resin is housed with 0.2mL / min flow velocity, obtains [ Emim]OH in water. According to the [Emim]OH: terephthalic acid molar ratio of 2:1, weigh an appropriate amount of terephthalic acid. [Emim]OH aqueous solution was added dropwise to terephthalic acid, and the reaction was stirred at 0°C for 24h. Afterwards, a large amount of water was removed by rotary evaporation at 60° C., and then placed in a vacuum drying oven for 24 hours at 60° C. to obtain the target ionic liquid.
Embodiment 2
[0034] This example illustrates that 1-propyl-3-methylimidazolium terephthalate ([Pmim] 2 TPA) preparation.
[0035] First prepare the 1-propyl group-3-methylimidazolium chloride salt ([Pmim]Cl) aqueous solution of 0.5mol / L, then this aqueous solution is slowly passed through the ion-exchange column that anion exchange resin is housed with 1mL / min flow velocity, obtains [ Pmim]OH in water. According to the [Pmim]OH: terephthalic acid molar ratio of 2:1, weigh an appropriate amount of terephthalic acid. [Pmim]OH aqueous solution was added dropwise to terephthalic acid, and the reaction was stirred at 3°C for 8h. Afterwards, a large amount of water was removed by rotary evaporation at 70° C., and then placed in a vacuum drying oven for 12 hours at 70° C. to obtain the target ionic liquid.
Embodiment 3
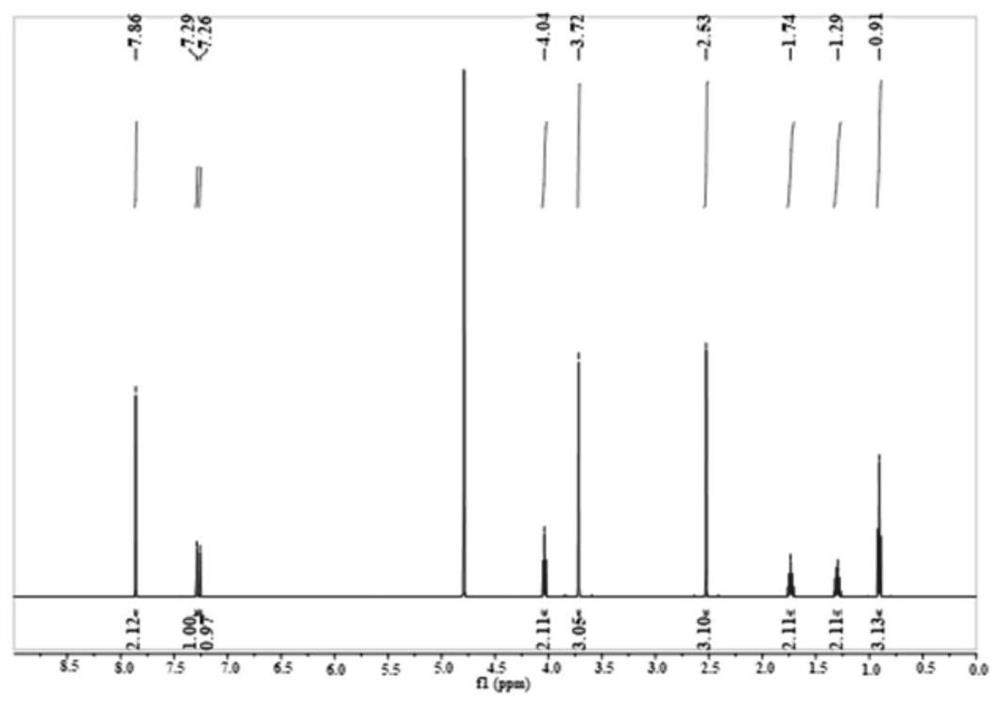
[0037] This example illustrates 1-butyl-3-methylimidazole terephthalate ([Bmim] 2 TPA) preparation.
[0038] First prepare the 1-butyl-3-methylimidazolium chloride salt ([Bmim]Cl) aqueous solution of 3mol / L, then this aqueous solution is slowly passed through the ion-exchange column that anion-exchange resin is housed with 0.1mL / min flow velocity, obtains [ Bmim]OH aqueous solution. According to the [Bmim]OH: terephthalic acid molar ratio of 2:1, weigh an appropriate amount of terephthalic acid. [Bmim]OH aqueous solution was added dropwise to terephthalic acid, and the reaction was stirred at 3°C for 24h. Afterwards, a large amount of water was removed by rotary evaporation at 80° C., and then placed in a vacuum drying oven for 48 hours at 80° C. to obtain the target ionic liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com