Immunotherapy for the treatment of cancer
A technology that regulates immunity and immune checkpoints, applied in the direction of immunoglobulin, antibody medical components, chemical instruments and methods, etc., to achieve the effect of inhibiting tumor growth and broadening efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0239] Example 1 - Effect of PEI-PEG-EGF / polyIC alone
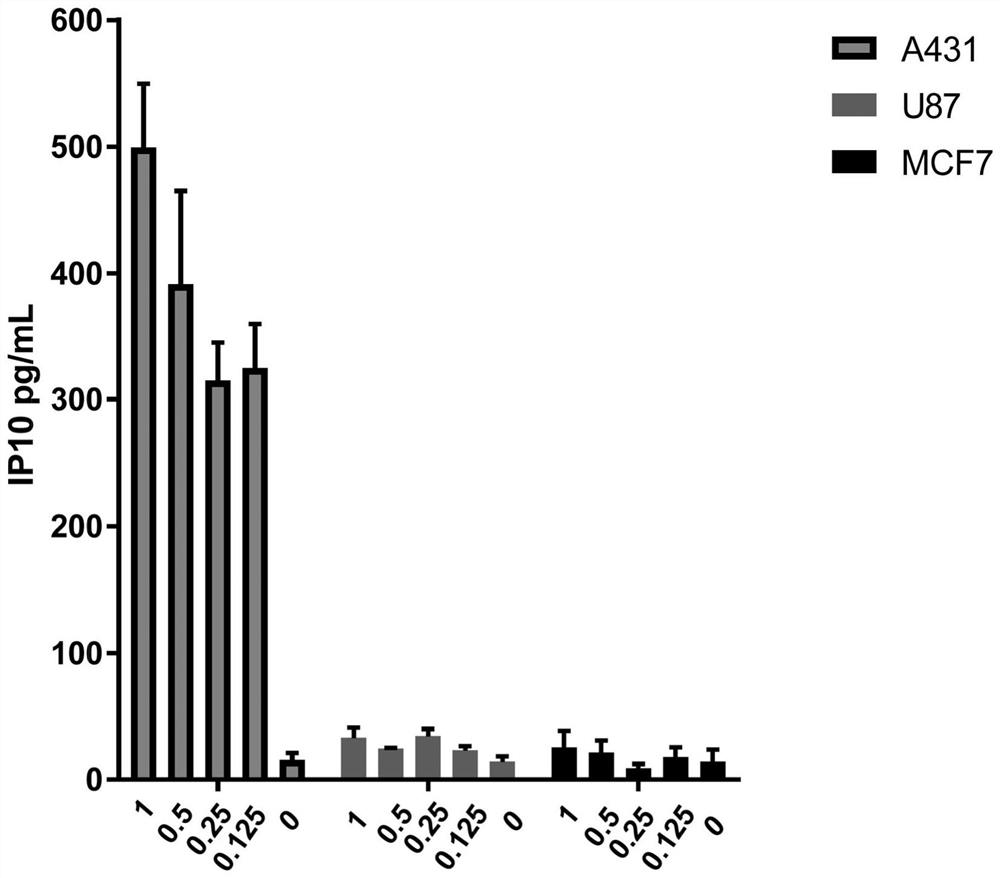
[0240] A431, U87 and MCF7 cells (40,000 cells per well) were treated with various concentrations (0.125, 0.25, 0.5, 1 μg / ml) of PEI-PEG-EGF / polyIC for 5 hours.
[0241] Human IP-10 (CXCL10) secretion was quantified by ELISA assay (ABTS ELISA Development Kit, Peprotech). IP10 secretion was strongly increased in A431 cells expressing high EGFR levels after incubation with PEI-PEG-EGF / polyIC for 5 hours ( figure 1 ).
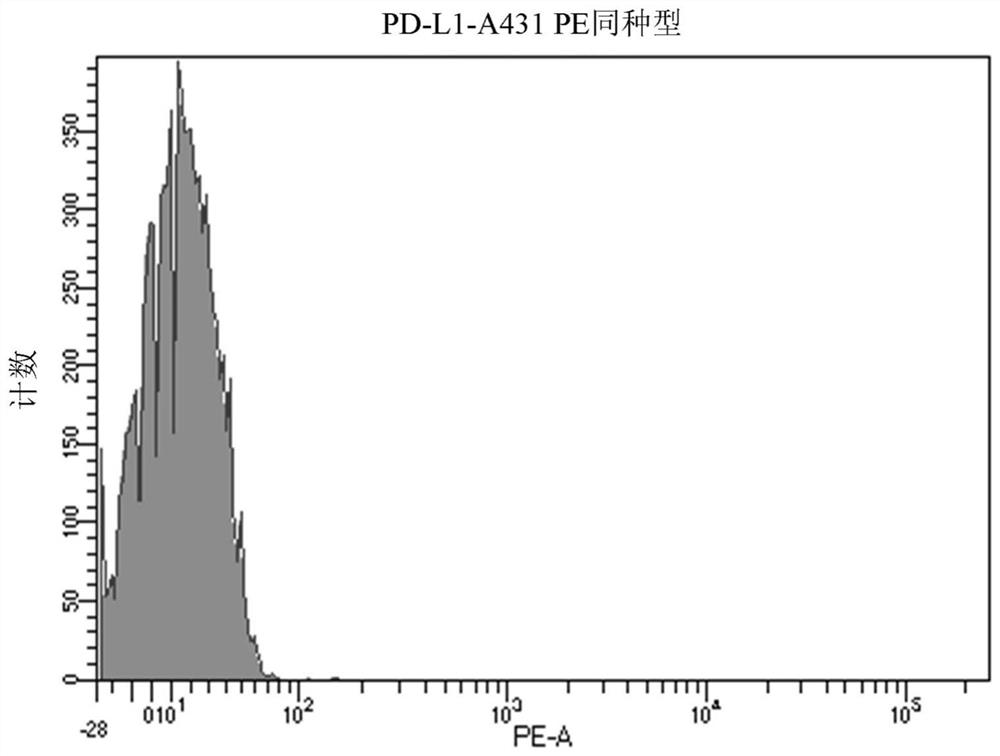
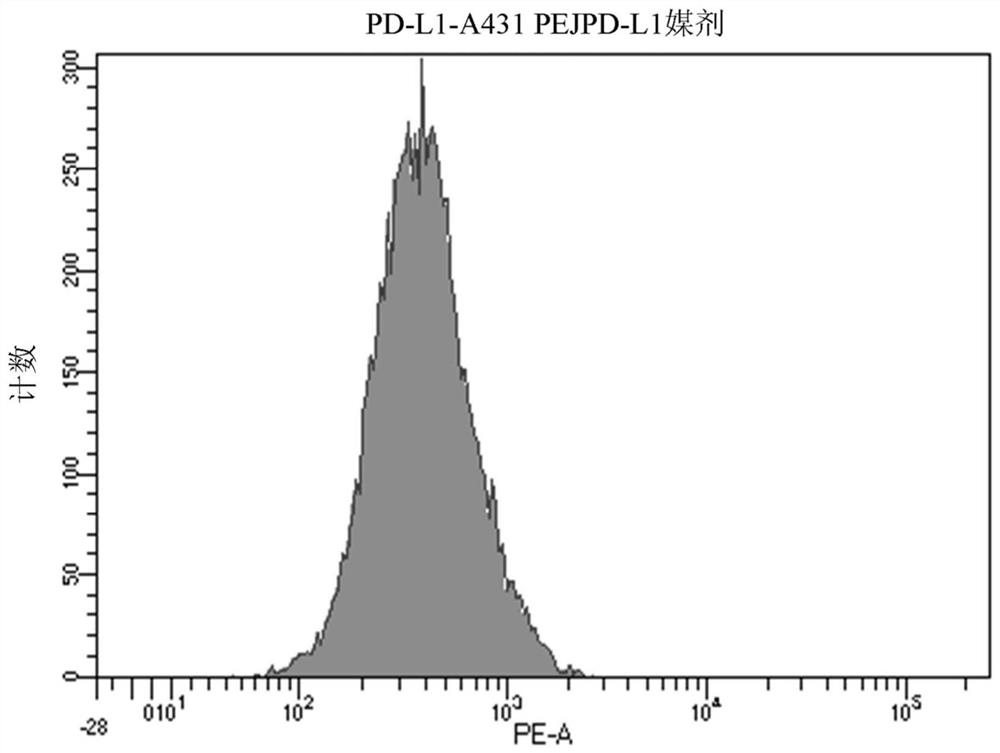
[0242] A431 cells were treated with PEI-PEG-EGF / polyIC at a concentration of 0.125 μg / ml for 5 hours. A431 cells were then stained with a PD-L1-PE-labeled antibody (Biolegend, Cat. No. 393607) in PBS with 2% FCS for 40 minutes on ice, washed and analyzed by FACS instrument. PD-L1 expression was significantly increased after PEI-PEG-EGF / polyIC treatment (MFI=751 ) compared to untreated control cells (MFI=431 ). An isotype control was used as a negative control (MFI=13).
[0243] After PEI-PEG-EGF / polyIC t...
example 2
[0246] Example 2 - In Vitro Effect of PEI-PEG-EGF / polyIC in Combination with Immunomodulatory Therapies Nivolumab and 4-1BB Antibody
[0247]Nivolumab: 40.000 A431 cells were treated with PEI-PEG-EGF / polyIC at concentrations of 0.125, 0.25, 0.5, 1 μg / ml for 5 hours. 200.000 PBMC were then stimulated with CD3 (5 μg / ml) and treated with diluted medium containing PEI-PEG-EGF / polyIC (0.125 μg / ml) alone or in combination with nivolumab (20 μg / ml) (1:2) to attack for 48 hours. After 48 hours, media from challenged PBMCs were collected and INFy production was measured by ELISA assay ( image 3 ).
[0248] Combining PEI-PEG-EGF / polyIC with nivolumab significantly increased IFN-γ production by PBMCs ( image 3 ).
[0249] Antibody 4-1BB: 40.000 A431 cells were treated with PEI-PEG-EGF / polyIC at a concentration of 0.5 μg / ml for 5 hours. 200,000 PBMCs from healthy donors were stimulated or unstimulated with CD3 (0.5 μg / ml) and compared with PEI-PEG-EGF / polyIC alone or with 4-1BB (Biol...
example 3
[0253] Example 3 - In vivo efficacy of the combination of PEI-PEG-mEGF / polyIC+anti-PD-1
[0254] The effect of PEI-PEG-EGF / polyIC+anti-PD-1 on RencaEGFR lung metastasis in immunocompetent mice was examined.
[0255] Materials and methods: cells: RencaEGFR; polyplex PEI-PEG-EGF / polyIC and -LPEI / EGF mice: 1 / 0.75); poly IC from Dalton; HBG (Hepes buffered glucose), N / P:8. Anti-PD-1: rat IgG2a, kappa anti-mouse PD-1 antibody (Bioxcell clone RMP1-14).
[0256] 250,000 RencaEGFR cells were injected intravenously into 40 Balb / c immunocompetent female mice (6 weeks old, weight: 18-21 gr) to induce RencaEGFR tumor formation in the lung. After 10 days, the animals were divided into 4 groups, 10 animals / group (untreated (UT), anti-PD-1, PEI-PEG-EGF / polyIC and PEI-PEG-EGF / polyIC+aPD1 (anti-PD -1).
[0257] Mice bearing RencaEGFR tumors were treated with PEI-PEG-EGF / polyIC alone at 250 μg / kg, 6 injections / week intravenously for 2 weeks, or with anti-PD-1 (RPM1-14, rat IgG2a, Biox cell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com