Covalent organic framework material as well as preparation method and application thereof
A covalent organic framework and organic solvent technology, applied in chemical instruments and methods, other chemical processes, iodine/hydrogen iodide, etc., can solve the problem of long iodine adsorption equilibrium time, achieve fast adsorption speed, strong adsorption capacity, The effect of channel order
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: The preparation method of the covalent organic framework material of this embodiment is carried out according to the following steps:
[0030] 1. Weigh 0.14g of 2-(4-aminophenyl)-1H-phenanthrene[9,10-d]imidazole-6,9-diamine and 0.21g of isophthalaldehyde respectively, and add them to the schlenk tube, Then add 1.0mL n-butanol and 0.05mL glacial acetic acid, mix well;
[0031] 2. The schlenk tube is evacuated and nitrogen-filled cycle operation, so that the nitrogen environment in the schlenk tube is maintained;
[0032] 3. Heat the schlenk tube to 115°C and react for 5 days;
[0033] 4. Cool down to room temperature after the reaction, filter with suction, wash the filter cake repeatedly with methanol, and dry in vacuum to obtain a covalent organic framework material with a yield of 78%.
[0034] In this embodiment, the schlenk tube is Shrek
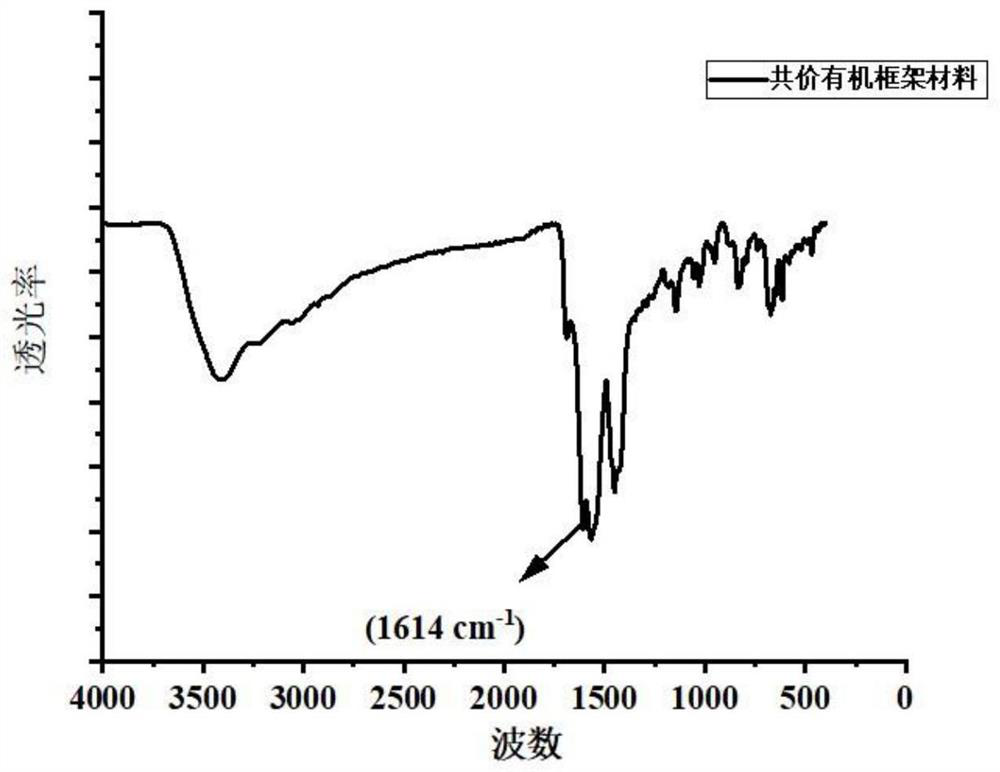
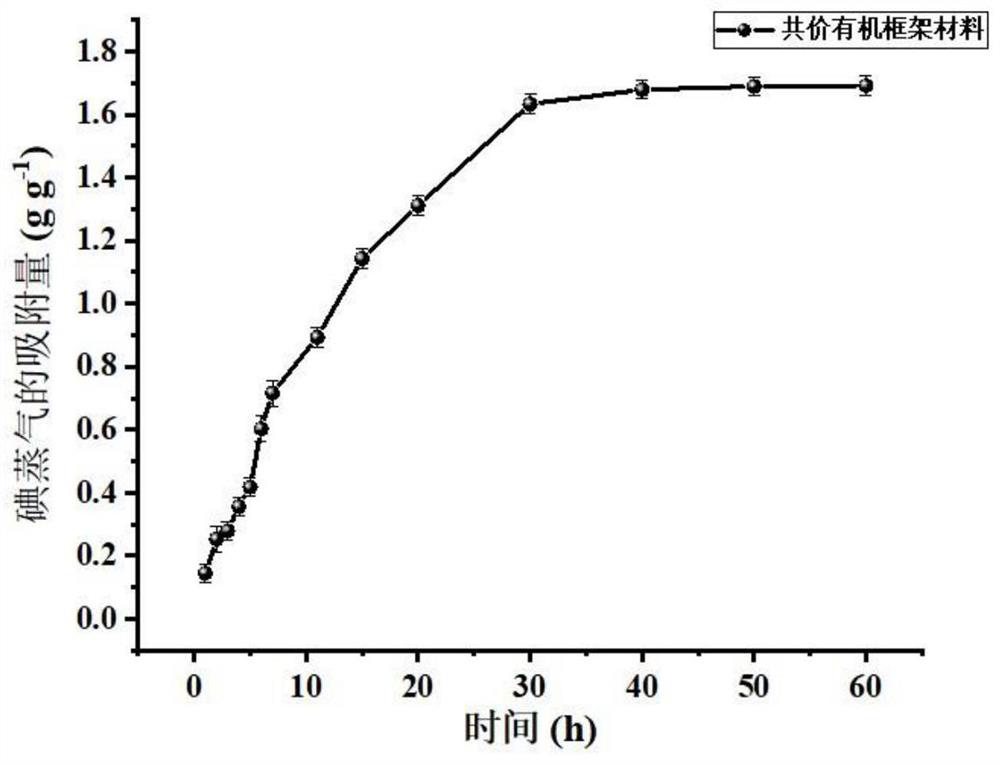
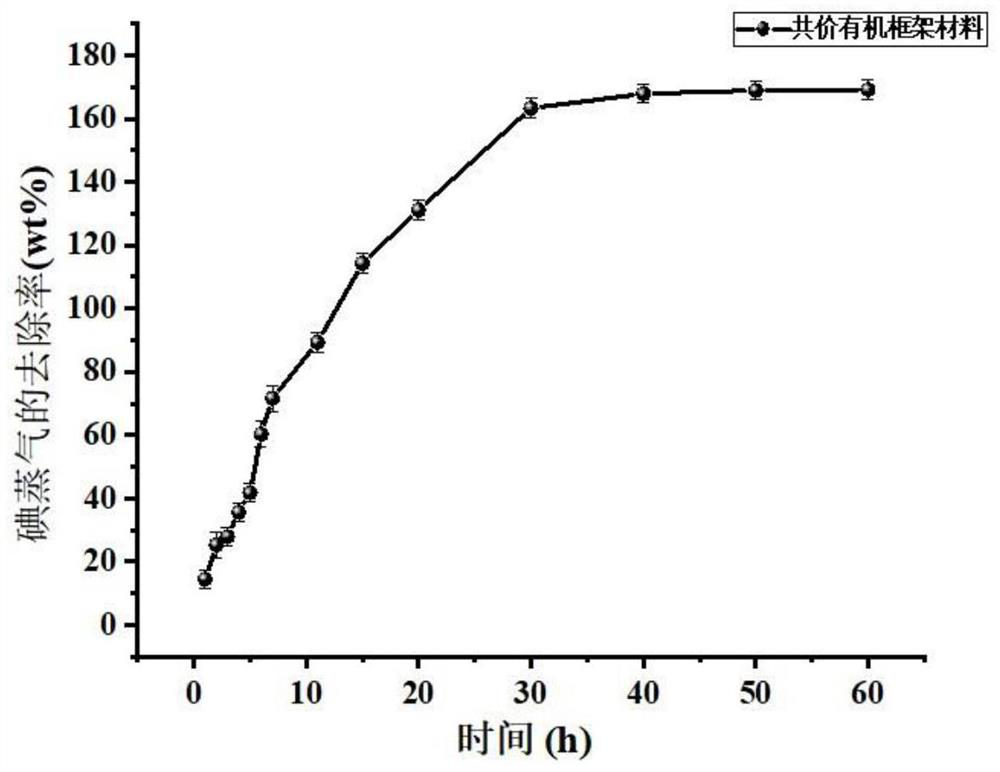
[0035] Structural characterization of the covalent organic framework material in Example 1 was carried out by F...
Embodiment 2
[0058] Embodiment 2: The preparation method of the covalent organic framework material of this embodiment is carried out according to the following steps:
[0059] 1. Weigh 0.14g of 2-(4-aminophenyl)-1H-phenanthrene[9,10-d]imidazole-6,9-diamine and 0.17g of m-phthalaldehyde respectively, and add them to the schlenk tube, Then add 1.0mL N,N-dimethylformamide and 0.05mL concentrated hydrochloric acid with a mass fraction of 37%, and mix well;
[0060] 2. The schlenk tube is evacuated and nitrogen-filled cycle operation, so that the nitrogen environment in the schlenk tube is maintained;
[0061] 3. Heat the schlenk tube to 150°C and react for 3 days;
[0062] 4. Cool down to room temperature after the reaction, filter with suction, wash the filter cake repeatedly with ethyl acetate, and dry in vacuum to obtain a covalent organic framework material, with a yield of 69%.
Embodiment 3
[0063] Embodiment 3: The preparation method of the covalent organic framework material of this embodiment is carried out according to the following steps:
[0064] 1. Weigh 0.14g of 2-(4-aminophenyl)-1H-phenanthrene[9,10-d]imidazole-6,9-diamine and 0.05g of m-phthalaldehyde respectively, and add them to the schlenk tube, Then add 0.5mL N,N-dimethylformamide, 0.5mL dioxane and 0.05mL concentrated sulfuric acid with a mass fraction of 98%, and mix well;
[0065] 2. The schlenk tube is evacuated and nitrogen-filled cycle operation, so that the nitrogen environment in the schlenk tube is maintained;
[0066] 3. Heat the schlenk tube to 130°C and react for 5 days;
[0067] 4. Cool down to room temperature after the reaction, filter with suction, wash the filter cake repeatedly with ethanol, and dry in vacuum to obtain a covalent organic framework material with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com