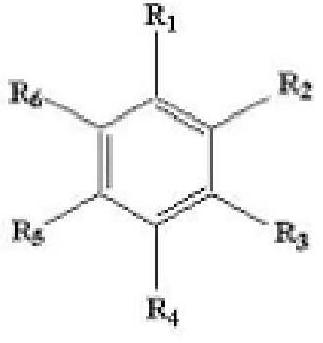
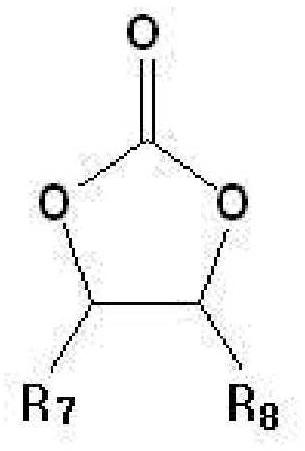
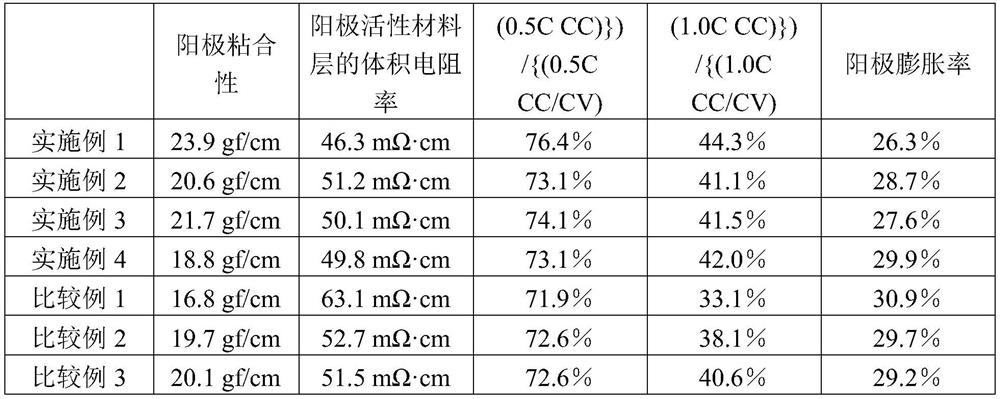
Anode binder material for lithium secondary battery, and anode binder comprising cured product thereof
A technology for rechargeable batteries and adhesives, applied in the field of 0003] The present invention relates to the field, and can solve problems such as side reactions, anode degradation, and inability to compensate for volume changes in anode active materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0147]61 g of butadiene, 57 g of styrene, and 2 g of acrylic acid were added as monomers to water containing 0.5% by weight of the polymerization initiator p-menthane hydroperoxide (PMHP), and 10 g of sodium lauryl sulfate was added as emulsified agents, and mixed them, and then polymerized at 70°C for about 5 hours to obtain a composition comprising butadiene-styrene-acrylic polymer particles.
[0148] The solids content in the composition was 30% by weight, and the polymer particles contained therein had a number average particle diameter of 50 nm (measured by a dynamic light scattering (DLS) device).
Embodiment 1
[0150] (1) Anode binder material (acrylic-styrene-butadiene polymer+sulfur+Zn(BDC)+DCBS+ZnO)
[0151] Get 20g of the composition of Preparation Example 1, and add 0.2g of sulfur (S 8 ), 0.4g of Zn(BDC) (wherein, BDC = 1,4-phthalate), 0.2g of DCBS (N,N-dicyclohexyl-2-benzothiazole sulfenamide) and 0.7g ZnO was then stirred for 1 hour to obtain the anode binder material of Example 1.
[0152] (2) anode
[0153] 150 g of a thickener, an aqueous carboxymethylcellulose solution (solid content: 1.5% by weight) and 1.5 g of a conductive agent acetylene black were mixed and stirred for 1 hour to prepare a conductive agent dispersion.
[0154] Take 0.5 g of the anode binder material of Example 1 and introduce it into the conductive agent dispersion, and introduce 150 g of the anode active material artificial graphite (D50: 20 μm) and 20 g of distilled water thereinto and stir to prepare the anode binder material of Example 1. Anode active material slurry.
[0155] Using a copper fo...
Embodiment 2
[0160] (1) Anode binder material (acrylic-styrene-butadiene polymer+sulfur+Zn(BDC)+DCBS+ZnO)
[0161] Get 20g of the composition of Preparation Example 1, and add 0.2g of sulfur (S 8 ), 0.4g of Zn(BDC), 0.1g of DCBS and 0.7g of ZnO, and then stirred for 1 hour to obtain the anode binder material of Example 2.
[0162] (2) Preparation of anode and lithium-ion half-cell
[0163] An anode and a lithium ion half cell of Example 2 were prepared by the same method as in Example 1, except that the anode binder material of Example 2 was used instead of the binder composition of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com