Purification method of high-purity fluorescein
A purification method and technology of fluorescein, which is applied in the field of fluorescein purification, can solve the problems that it is difficult to obtain high-purity fluorescein, and achieve the effects of easy reduction, easy preparation, and good crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
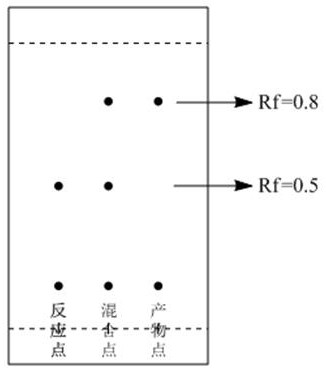
[0042] The crude fluorescein obtained by co-heating phthalic anhydride and resorcinol is used as a raw material. The purity of the raw material is not limited, and the conventional crude fluorescein obtained in industry is sufficient. Take 25g of crude fluorescein and 125ml of glacial acetic acid in a 500mL three-necked reaction bottle, stir evenly at 80-100 rpm, then take a sample and spot the plate to obtain the following: figure 1 For the reaction point shown, continue to add 5g of pyridine (acid-binding agent) and 10g of acetic anhydride into the reaction bottle, control the temperature at 50-60°C and stir the reaction for 3h at a speed of 80-100rpm, and continue to take samples and spot the plate to obtain the following: figure 1 As shown in the product point, TLC monitors developing agent 1ml ethyl acetate plus 2ml petroleum ether, diacetyl fluorescein Rf = 0.8, the reactant crude fluorescein Rf = 0.5, the product point has no crude fluorescein, it proves that the reactio...
Embodiment 2
[0048] The parts of this embodiment that are not specifically described are consistent with Embodiment 1.
[0049]Take 100g of crude fluorescein and 500ml of glacial acetic acid in a 1L three-necked reaction flask, stir evenly, add 20g of pyridine and 40g of acetic anhydride, stir at a temperature of 50-60°C for 3 hours, monitor the reaction result by TLC, add 2ml of ethyl acetate to 1ml of developer Petroleum ether produces diacetyl fluorescein Rf = 0.8, and the crude fluorescein Rf = 0.5. After the reaction was complete, the temperature was lowered to below 25°C, stirred and crystallized for 1 hour, and suction filtered. The filter cake was beaten once with 500ml of purified water, and dried at 50°C by blowing air to obtain 112g of orange-yellow diacetylfluorescein. Dissolve with 5 times the volume of dichloromethane at 40°C, add 5 times the volume of diethyl ether to precipitate, cool to 0-5°C and stir for 1 hour, then filter with suction, and air-dry at 50°C to obtain 101 ...
Embodiment 3
[0054] The parts of this embodiment that are not specifically described are consistent with Embodiment 1.
[0055] Take 3kg of crude fluorescein and 15L of glacial acetic acid in a 50L glass reactor, stir evenly, add 600g of pyridine and 1.2kg of acetic anhydride, stir at a temperature of 50-60°C for 3 hours, monitor the reaction results by TLC, add 1ml of ethyl acetate as a developer 2ml of petroleum ether produces diacetyl fluorescein Rf=0.8, and the crude fluorescein Rf=0.5. After the reaction was complete, the temperature was lowered to below 25°C, stirred and crystallized for 1 hour and suction filtered. The filter cake was beaten once with 15 L of purified water, and air-dried at 50°C for 8 hours to obtain 3.40 kg of orange-yellow diacetyl fluorescein. Dissolve with 5 times the volume of dichloromethane at 40°C, add 5 times the volume of diethyl ether to precipitate, cool to 0-5°C and stir for 1 hour, then filter with suction, and air-dry at 50°C to obtain 3.05kg of whit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com