Preparation method of acesulfame potassium
A technology of acesulfame potassium and sulfamic acid, applied in chemical instruments and methods, physical/chemical process catalysts, organic chemistry, etc., can solve the problem of low yield of finished products of acesulfame potassium, easy decomposition of cyclization products, and hydrolysis reactions Low yield and other problems, to achieve the effect of improving acylation reaction efficiency, reducing production cost and increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of acesulfame potassium provided by the application at least includes step S110 to step A140:
[0032] Intermediate preparation step S110: adding triethylamine to the sulfamic acid solution for amination reaction to generate a sulfamic acid ammonium salt solution; , carry out the acylation reaction to obtain the intermediate solution.
[0033] The preparation of the intermediate is more carefully divided into two small steps. First, it is the preparation of ammonium sulfamate, and then the intermediate is prepared by reacting ammonium sulfamate with diketene, that is, acetoacetamide-N-sulfonic acid triethyl amine salt.
[0034] The ammonium salt of sulfamic acid is obtained by adding triethylamine to the sulfamic acid solution for amination reaction. Specifically, in some embodiments of the present application, sulfamic acid is dissolved in the first solvent to configure the first reaction solution; triethylamine is dissolved in the second solve...
Embodiment 1
[0073] Example 1 (Example 1A, Example 1B, Example 1C, Example 1D, Example 1E, Example 1F, Example 1G, Example 1H)
[0074]Preparation of intermediate solution: When preparing intermediate solution, use super acid SO 4 2- / Fe 2 o 3 As a catalyst, other steps refer to the preparation of intermediate solution A.
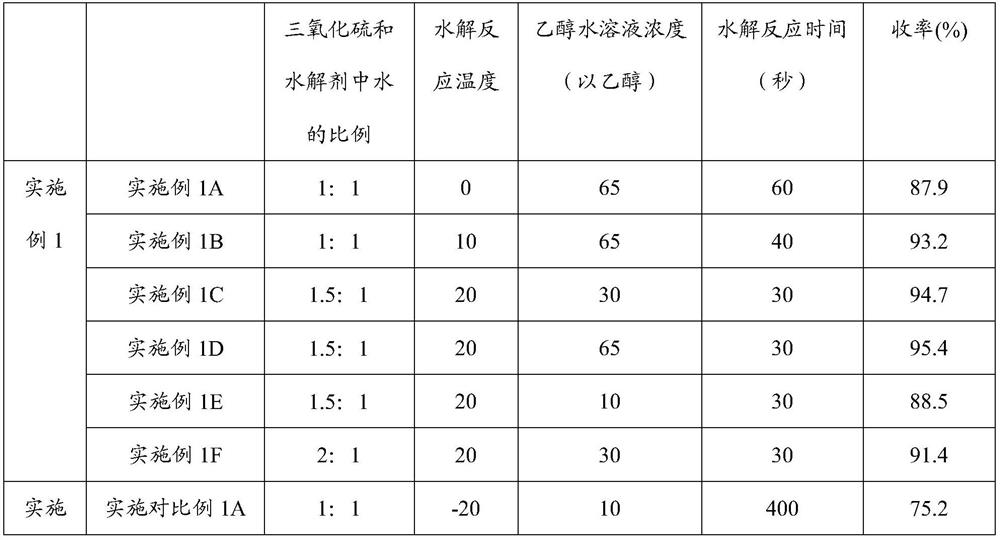
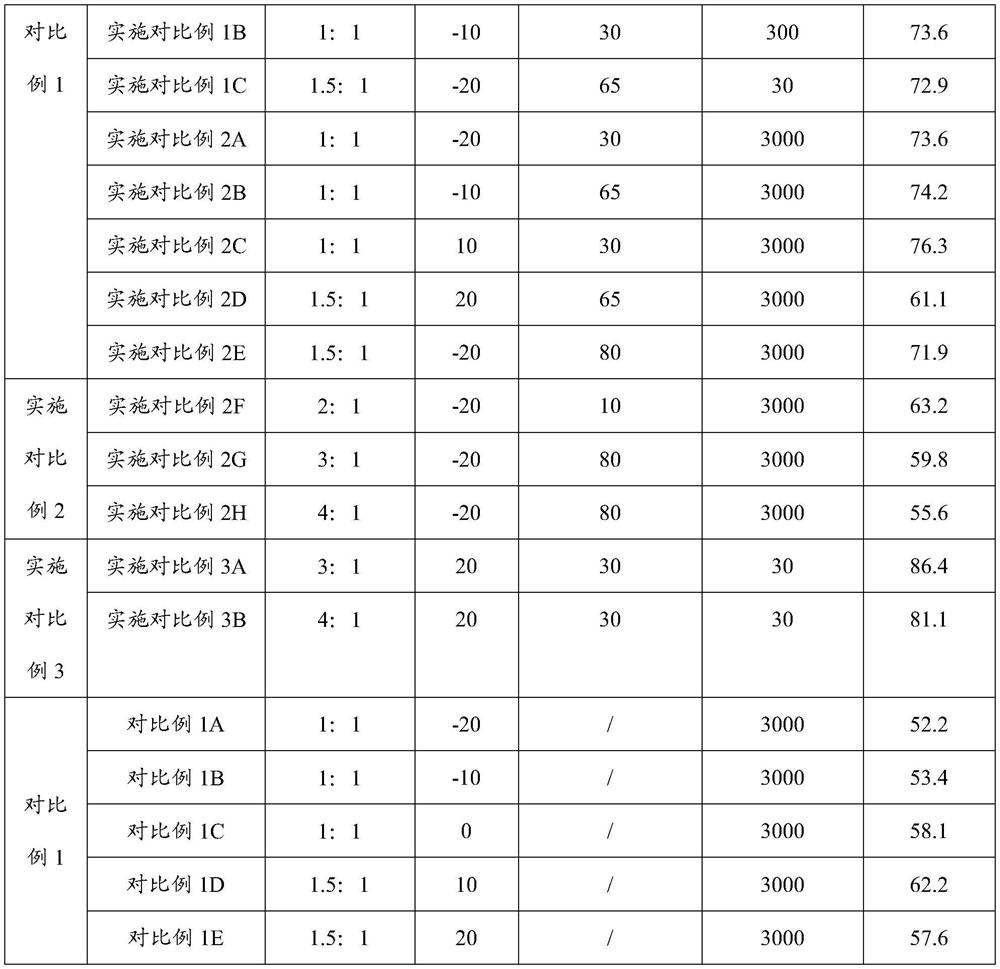
[0075] Sulfonation and cyclization step: dissolving sulfur trioxide in dichloromethane to form a cyclization agent solution; adding the cyclization agent solution to the intermediate solution to carry out sulfonation and cyclization reaction to obtain a cyclization product solution.
[0076] Hydrolysis step: adding ethanol aqueous solution as a hydrolysis agent to the cyclization product solution to carry out hydrolysis reaction to obtain a hydrolyzate solution.
[0077] Salt-forming step: adding an ethanol solution of potassium hydroxide to the organic phase of the hydrolyzate solution to obtain acesulfame potassium. Please refer to Table 1 for specific reaction c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com