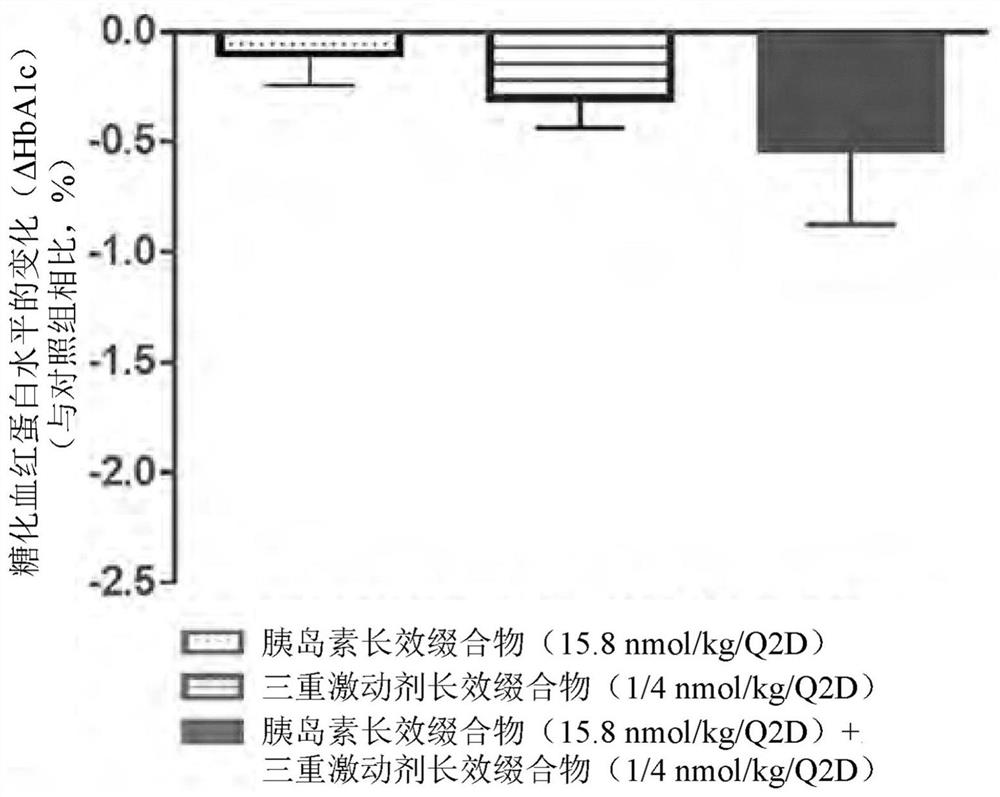
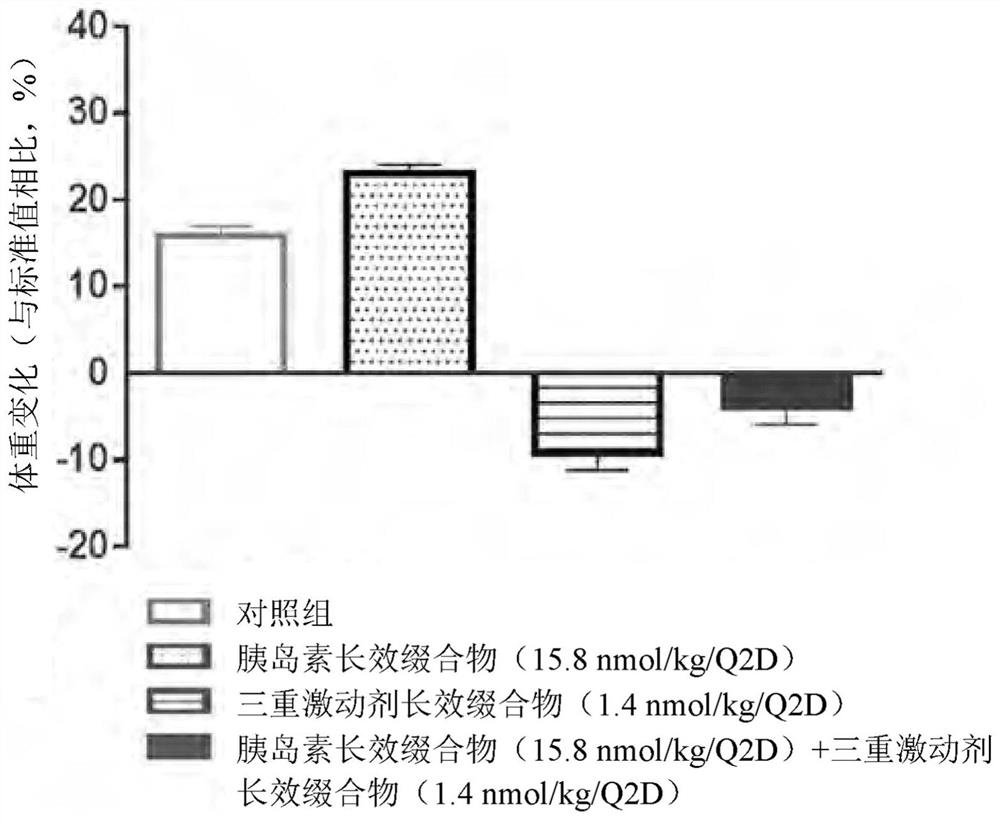
Pharmaceutical composition comprising insulin and triple agonist having activity with respect to all of glucagon and glp-1 and gip receptor
A technology of glucagon and GLP-1, applied in the field of compound preparations, can solve the problems of combined use of combination preparations, etc., and achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0693] Example 1: Preparation of triple agonists and long-acting conjugates thereof
[0694] (1) Preparation of triple agonist
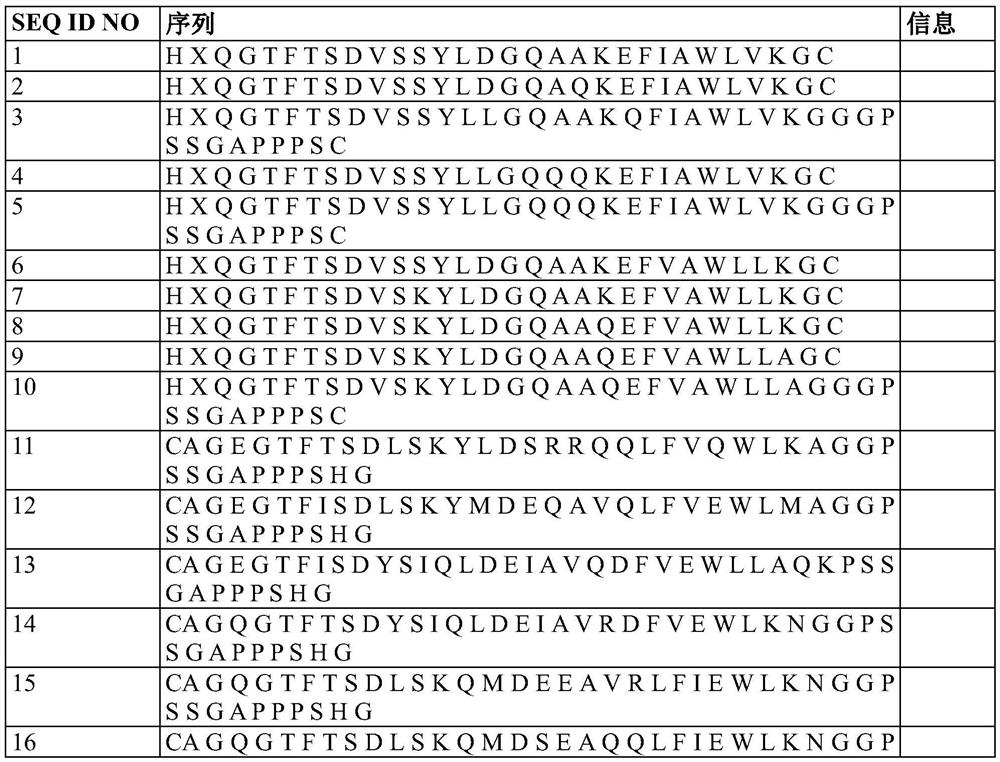
[0695] Triple agonists exhibiting activity at all of the glucagon, GLP-1 and GIP receptors were prepared and their sequences are shown in Table 1 below.
[0696] Table 1
[0697]
[0698]
[0699]
[0700]
[0701] In the sequences shown in Table 1, amino acids marked with X represent aminoisobutyric acid (Aib), which is an unnatural amino acid, and underlined amino acids represent amino acids that form a loop together. In Table 1, CA represents 4-imidazole acetyl, and Y represents tyrosine.
[0702] (2) Preparation of triple agonist long-acting conjugates
[0703] In order to make 10kDa PEG with maleimide group and aldehyde group at its two ends, namely maleimide-PEG-aldehyde (10kDa, NOF, Japan) and triple agonist (SEQ ID NO:21 , 22, 42, 43, 50, 77 and 96) cysteine residues PEGylated such that the triple agonist and maleimide-PEG...
example 2
[0713] Example 2: Preparation of native insulin long-acting conjugates
[0714] To pegylate 3.4K propion-ALD(2)PEG (3.4kDa PEG with one propionaldehyde group at each end, NOF, Japan) at the N-terminus of the B-chain of human native insulin (India, Biocon), natural Insulin and PEG were reacted at 25°C for 2 hours at a molar ratio of 1:4, where the native insulin concentration was 5 mg / mL. By adding 3 mM sodium cyanoborohydride (NaCNBH) as a reducing agent to a mixed solvent of 50 mM sodium citrate buffer (pH5. 3 ) to react. The reaction solution was purified using a SP-HP (GE Healthcare) column using a buffer containing sodium citrate (pH 3.0) and 45% EtOH and a KCl concentration gradient.
[0715] Next, in order to attach native insulin-linked PEG to the N-terminus of the immunoglobulin Fc fragment, the purified mono-PEGylated insulin and the immunoglobulin Fc fragment were reacted at a molar ratio of 1:1.2 at 25°C for 15 hours, At the same time, the total protein concent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| weight change | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com