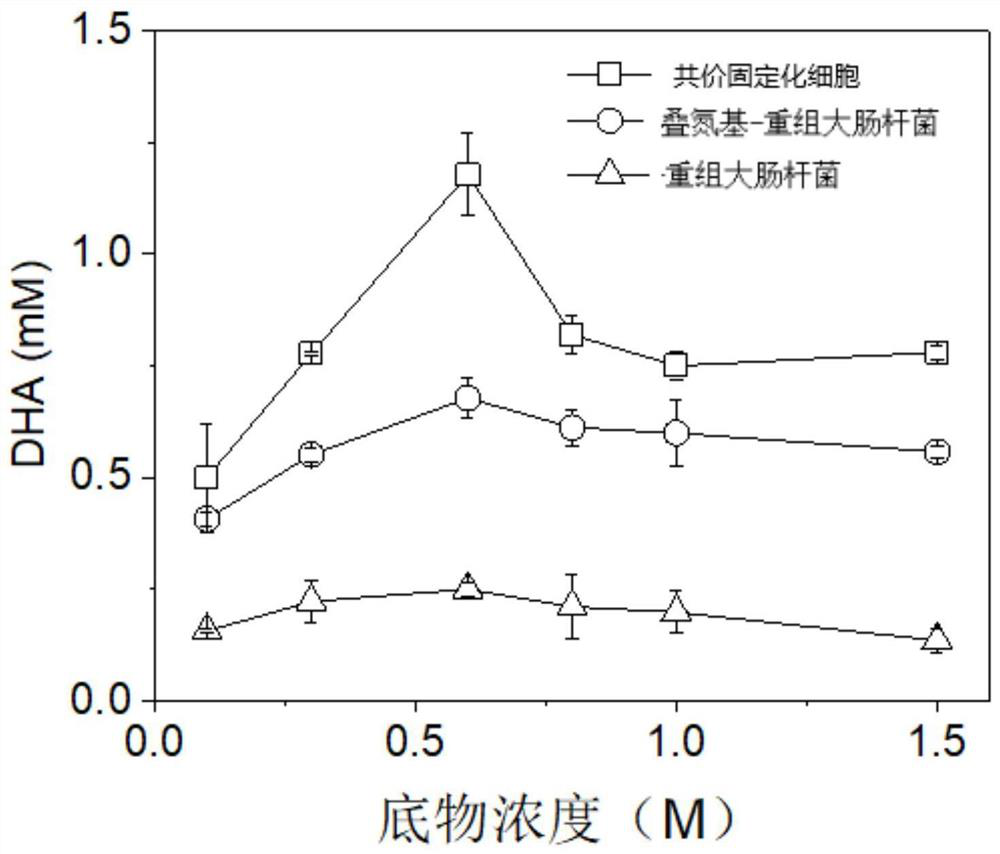
Covalent immobilized cell as well as preparation method and application thereof
An immobilized cell and covalent technology, applied in the field of bioengineering, can solve the problems of inapplicability to large-scale production, difficulty in separation and purification, and difficulty in repeated use, and achieve good biodegradability, good biocompatibility, and guaranteed enzyme The effect of the activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of covalently immobilized cells
[0035] (1) Preparation of alkyne-functionalized magnetic nanoparticles:
[0036] Fe 2+ and Fe 3+ Preparation of Fe by co-precipitation method at a ratio of 1:3 3 o 4 Magnetic nanoparticles, take 1g Fe 3 o 4 Nanoparticles 3 mL tetraethyl silicate, stirred for 5 hours to obtain Fe 3 o 4 @SiO 2 nanoparticles.
[0037] to 0.5 mg Fe 3 o 4 @SiO 2 Add 10 mL of 3-aminopropyltriethoxysilane to the nanoparticles, stir vigorously for 24 hours in the anaerobic environment, then add 400 μL of propiolic acid, and react overnight in the anaerobic condition to obtain alkyne-functionalized magnetic Nanoparticles, denoted as Fe 3 o 4 @SiO 2 -NH 2 -alkyne.
[0038] (2) Preparation of recombinant Escherichia coli after azido modification:
[0039] Recombinant Escherichia coli containing the glycerol dehydrogenase gene in KDO-N 3 Cultured in culture medium, adding IPTG with a final concentration of 0.1 mM to induce ex...
Embodiment 2
[0043] Example 2: Preparation of covalently immobilized cells
[0044] (1) Preparation of alkyne-functionalized magnetic nanoparticles:
[0045] Fe 2+ and Fe 3+ Preparation of Fe by co-precipitation method at a ratio of 1:3 3 o 4 Magnetic nanoparticles, take 3 g Fe 3 o 4 Nanoparticles 9 mL tetraethyl silicate, stirred for 5 hours to obtain Fe 3 o 4 @SiO 2 nanoparticles.
[0046] to 7.5 mg Fe 3 o 4 @SiO 2 Add 15 mL of 3-aminopropyltriethoxysilane to the nanoparticles, stir vigorously for 24 hours in the anaerobic environment, then add 600 μL of propiolic acid, and react overnight in the anaerobic condition to obtain alkyne-functionalized magnetic Nanoparticles, denoted as Fe 3 o 4 @SiO 2 -NH 2 -alkyne.
[0047] (2) Preparation of recombinant Escherichia coli after azido modification:
[0048] Recombinant Escherichia coli containing the glycerol dehydrogenase gene in KDO-N 3 cultured in culture medium, adding IPTG at a final concentration of 0.5 mM to induce e...
Embodiment 3
[0052] Example 3: Preparation of covalently immobilized cells
[0053] (1) Preparation of alkyne-functionalized magnetic nanoparticles:
[0054] Fe 2+ and Fe 3+ Preparation of Fe by co-precipitation method at a ratio of 1:3 3 o 4 Magnetic nanoparticles, take 5 g Fe 3 o 4 Nanoparticles 15 mL tetraethyl silicate, stirred for 5 hours to obtain Fe 3 o 4 @SiO 2 nanoparticles.
[0055] to Fe 3 o 4 @SiO 2 Add 3-aminopropyltriethoxysilane to the nanoparticles, stir vigorously for 24 hours in anaerobic environment, then add 800 μL propiolic acid, and react overnight in anaerobic conditions to obtain alkyne-functionalized magnetic nanoparticles , denoted as Fe 3 o 4 @SiO 2 -NH 2 -alkyne.
[0056] (2) Preparation of recombinant Escherichia coli after azido modification:
[0057] Recombinant Escherichia coli containing the glycerol dehydrogenase gene in KDO-N 3 cultured in culture medium, adding IPTG with a final concentration of 1 mM to induce expression at 25°C for 6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com