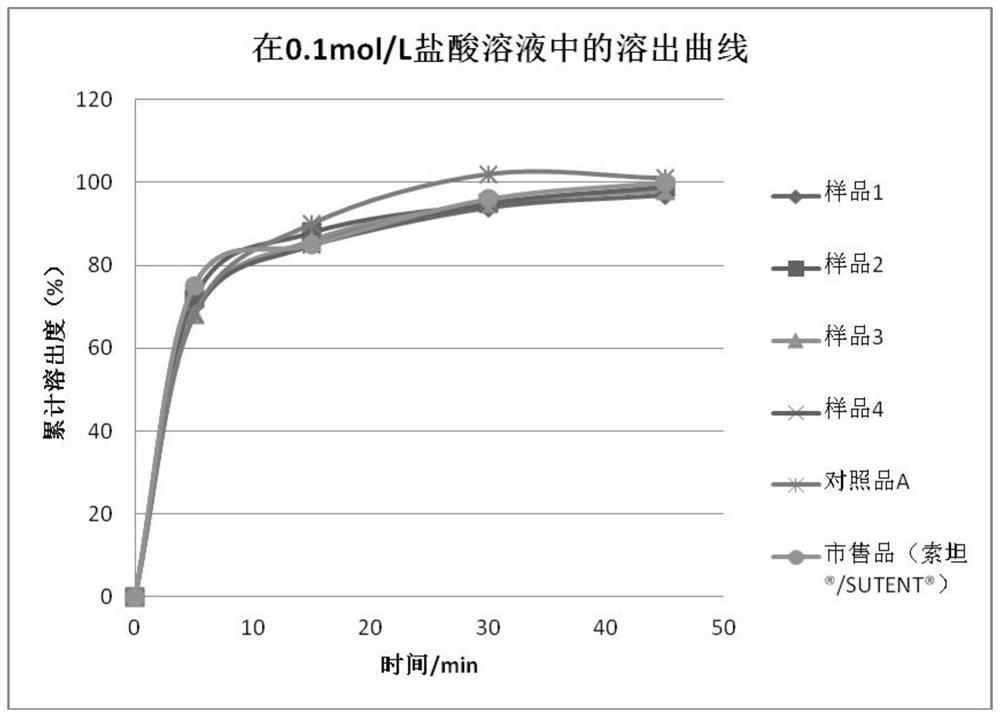
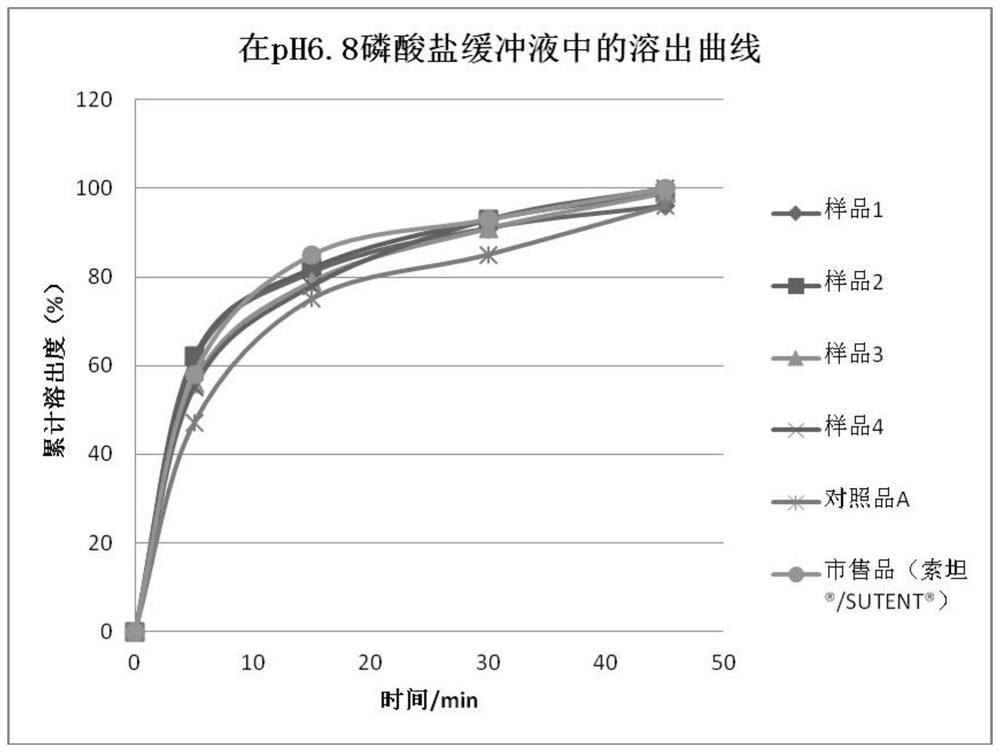
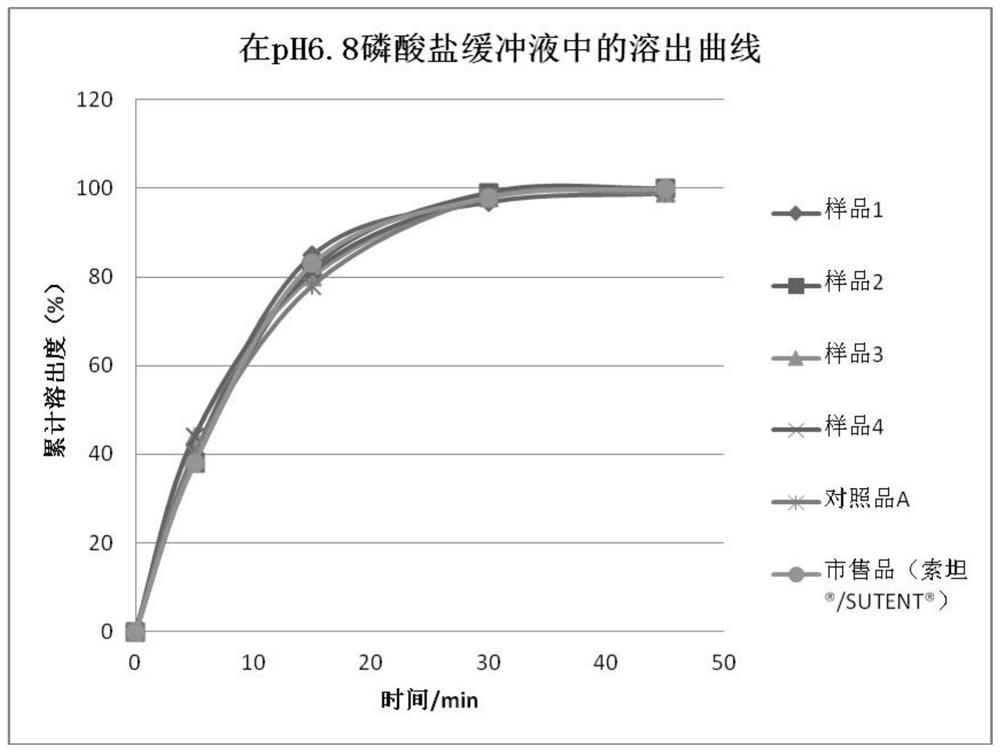
Sunitinib malate capsules and preparation method thereof
A technology of sunitinib malate capsules and sunitinib malate, applied in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as increasing operating costs of enterprises, and reduce operating costs Cost, high safety, low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Table 1 Recipe 1
[0039]
[0040] The preparation method of sunitinib malate capsules:
[0041] ①. Treatment of raw and auxiliary materials: Screen the raw materials of sunitinib malate to control the particle size of sunitinib malate to be greater than 30 μm (D90); pass colloidal silicon dioxide and magnesium stearate through a 60-mesh sieve respectively .
[0042] 2. Premixing: get sunitinib malate, starch hydrolyzed oligosaccharide, trehalose, and colloidal silicon dioxide of the recipe, place them in a three-dimensional mixer, and control the rotating speed during mixing to be 15 rpm, and the mixing time is 10min.
[0043] 3. Total mixing: add magnesium stearate into the three-dimensional mixer for further mixing, control the rotating speed during mixing to 15 rpm, and the mixing time to 5 min.
[0044] ④. Filling capsules: Fill the mixed materials in the three-dimensional mixer into capsules.
[0045] ⑤. Packaging: It is packaged in aluminum foil / PVC / Aclar ...
Embodiment 2
[0047] Table 2 Recipe 2
[0048]
[0049]
[0050] The preparation method of sunitinib malate capsules:
[0051] ①. Treatment of raw and auxiliary materials: Screen the raw materials of sunitinib malate to control the particle size of sunitinib malate to be greater than 30 μm (D90); pass colloidal silicon dioxide and magnesium stearate through a 60-mesh sieve respectively .
[0052] 2. Premixing: get sunitinib malate, starch hydrolyzed oligosaccharide, trehalose, and colloidal silicon dioxide of the recipe, place them in a three-dimensional mixer, and control the rotating speed during mixing to be 15 rpm, and the mixing time is 10min.
[0053] 3. Total mixing: add magnesium stearate into the three-dimensional mixer for further mixing, control the rotating speed during mixing to 15 rpm, and the mixing time to 5 min.
[0054] ④. Filling capsules: Fill the mixed materials in the three-dimensional mixer into capsules.
[0055] ⑤. Packaging: It is packaged in aluminum foi...
Embodiment 3
[0057] Table 3 Recipe 3
[0058]
[0059]
[0060] The preparation method of sunitinib malate capsules:
[0061] ①. Treatment of raw and auxiliary materials: Screen the raw materials of sunitinib malate to control the particle size of sunitinib malate to be greater than 30 μm (D90); pass colloidal silicon dioxide and magnesium stearate through a 60-mesh sieve respectively .
[0062] 2. Premixing: get sunitinib malate, starch hydrolyzed oligosaccharide, trehalose, and colloidal silicon dioxide of the recipe, place them in a three-dimensional mixer, and control the rotating speed during mixing to be 15 rpm, and the mixing time is 10min.
[0063] 3. Total mixing: add magnesium stearate into the three-dimensional mixer for further mixing, control the rotating speed during mixing to 15 rpm, and the mixing time to 5 min.
[0064] ④. Filling capsules: Fill the mixed materials in the three-dimensional mixer into capsules.
[0065]⑤. Packaging: It is packaged in aluminum foil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com