Cytisine tablet and preparation method thereof
A technology of cytisine tablets and cytisine, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low bioavailability, and achieve tablet The effect of controllable quality and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
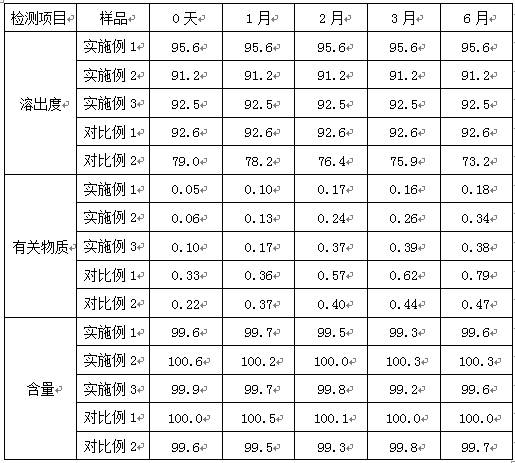
Examples
Embodiment 1
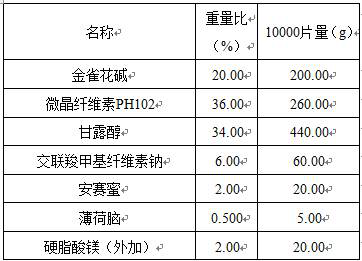
[0006] A kind of cytisine tablet, is made of the component that comprises following percentage by weight:
[0007]
[0008] (1) Pretreatment of raw and auxiliary materials: crush the raw material cytisine through a 200-mesh sieve; the particle size of the raw material is 20 μm;
[0009] (2) Mixing: Mix cytisine and filler disintegrant for 10 to 30 minutes to make them uniform;
[0010] (3) Add the prescribed amount of magnesium stearate to the powder mixed uniformly in step (2), and mix for 3 to 5 minutes to make it uniform;
[0011] (4) Directly compress the uniformly mixed drug powder in step (3), and control the hardness within the range of 50N to 80N.
Embodiment 2
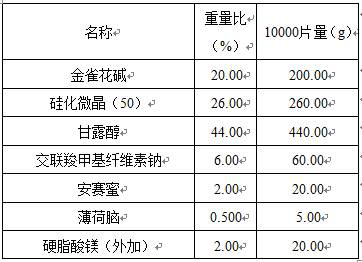
[0013] A kind of cytisine tablet, is made of the component that comprises following percentage by weight:
[0014]
[0015] (1) Pretreatment of raw and auxiliary materials: crush the raw material cytisine and pass through a 200-mesh sieve; the particle size of the raw material is 26 μm;
[0016] (2) Mixing: Mix cytisine and filler disintegrant for 10 to 30 minutes to make them uniform;
[0017] (3) Add the prescribed amount of magnesium stearate to the powder mixed uniformly in step (2), and mix for 3 to 5 minutes to make it uniform;
[0018] (4) Directly compress the uniformly mixed drug powder in step (3), and control the hardness within the range of 50N to 80N.
Embodiment 3
[0020] A kind of cytisine tablet, is made of the component that comprises following percentage by weight:
[0021]
[0022] (1) Pretreatment of raw and auxiliary materials: crush the raw material cytisine through a 200-mesh sieve; the particle size of the raw material is 20 μm;
[0023] (2) Mixing: Mix cytisine and filler disintegrant for 10 to 30 minutes to make them uniform;
[0024] (3) Add the prescribed amount of magnesium stearate to the powder mixed uniformly in step (2), and mix for 3 to 5 minutes to make it uniform;
[0025] (4) Directly compress the uniformly mixed drug powder in step (3), and control the hardness within the range of 50N to 80N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet weight | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com