Transaminase and application thereof in preparation of sitagliptin
A transaminase and sequence technology, applied to transaminase and its application field in the preparation of sitagliptin, can solve the problems of poor tolerance of transaminase, no catalytic activity or low catalytic activity, poor stability of free transaminase, etc., and achieves organic solvent resistance. Good receptivity, favorable for separation and purification, and ideal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: Screening a transaminase and constructing a recombinant expression vector comprising a transaminase coding gene
[0076] 1.1 screening aminotransferase
[0077] The soil samples of 5 to 15 cm were collected in a paddy field in Chang'an Town, Haining City, Hangzhou, as a screening sample as a transaminase. Weigh 5g soil samples and placed in 50 ml of centrifuge tube, then add 5 mL of 0.1% (w / w) sodium phosphate solution, reversal of mixing for 20 min after mixing, where each 10 min is gently Upside down; then, the supernatant was removed at room temperature under room temperature, and the supernatant was removed to collect the precipitate; weigh 0.5 g of precipitate, using a FASTDNA kit to symmetled genomic extraction, obtain soil Genome.
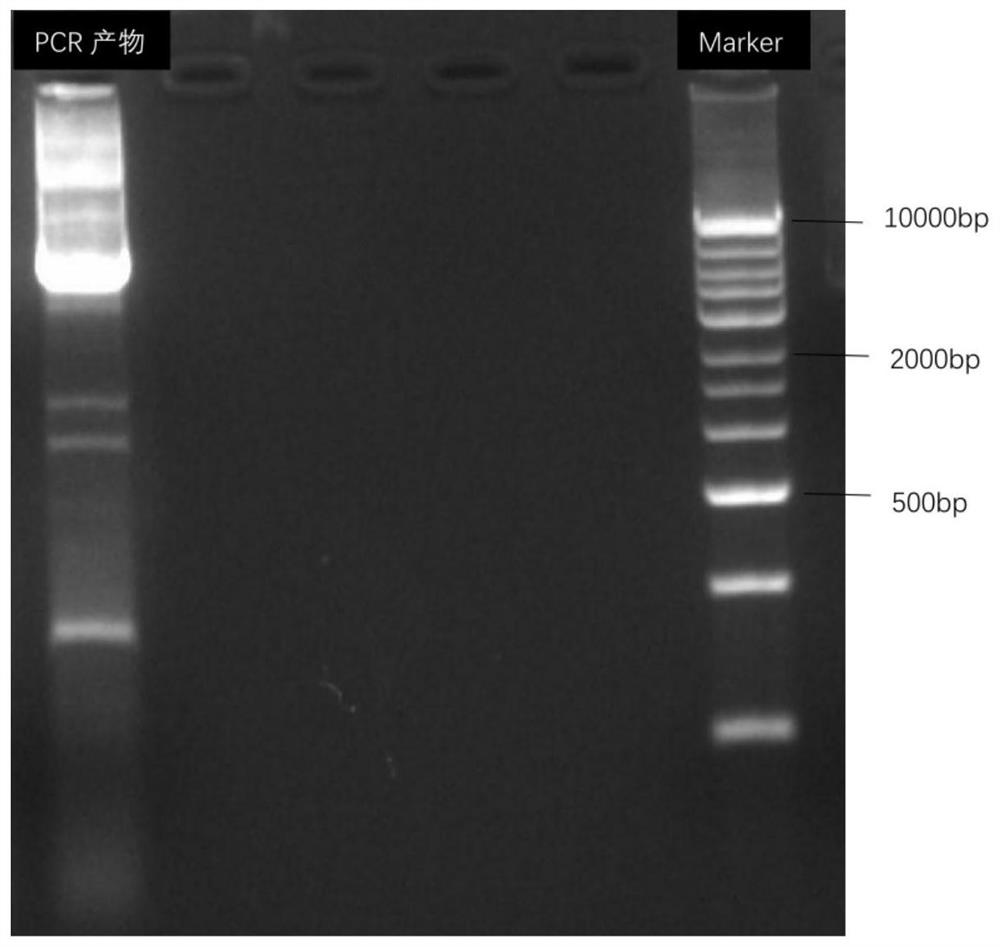
[0078] The PCR primer to the F1 (upstream primer) and R1 (downstream primer) according to the conservative amino acid sequence upstream of the transaminase, and add the enzyme-cut position BamH I and NDE I and NDE I, wherein ...
Embodiment 2
[0085] Example 2 Construction of a genetic engineering containing a transaminase encoding gene
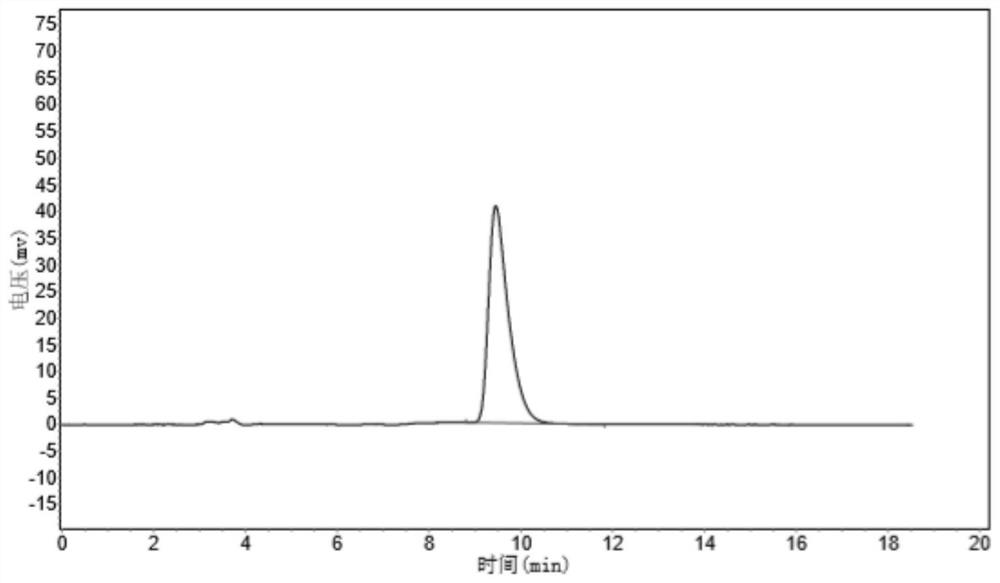
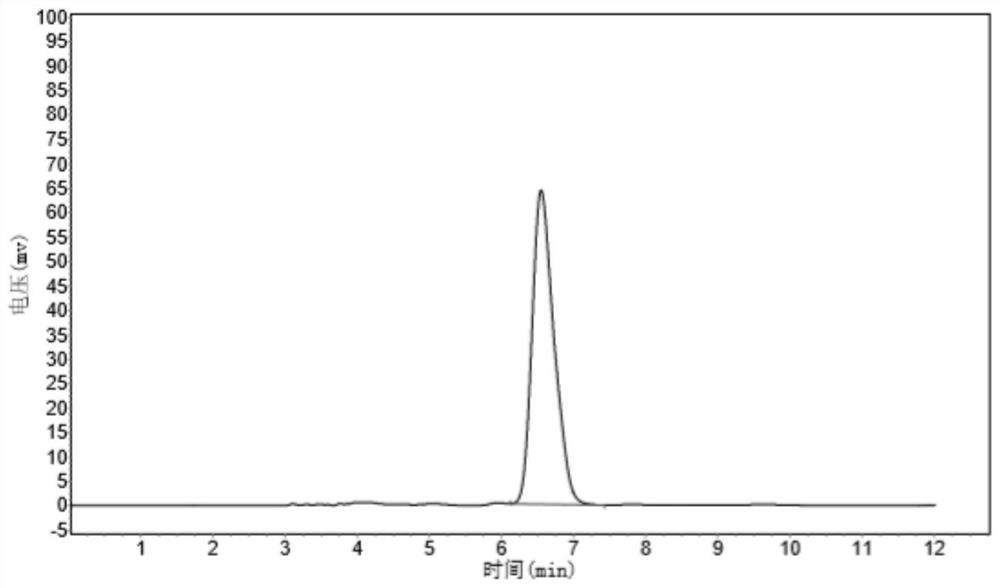
[0086] A transaminase having a amino acid sequence as shown in SEQ ID NO: 1 will be mutated to obtain a plurality of transaminase mutants, wherein the specific mutation is shown in Table 2.
[0087] Using the fixed-point mutation strategy, OligO7 software is designed to design a mutant primer based on the mutant amino acid sites to be mutated, and mutations are introduced by insertion, replace or deleting bases in a 5 'end of the upper and downstream mutant primers. The recombinant expression vector PUC19-WT constructed in Example 1 was selected as a template, and the KOD high fidel enzyme kit was used to reverse PCR, thereby obtaining a transaminase mutation sequence. The transaminase mutation sequence obtained by DPN I restriction endonuclease treatment, the enzyme-digestive product was connected to the T4 ligase, and the E. coli BL21 (DE3) was converted, and then the lb resistance p...
Embodiment 3
[0090] Example 3 induction expression and post-treatment of genetic engineering containing transaminase coding genes
[0091] The genes obtained in Example 2 were inoculated into an LB liquid medium containing 50 μg / ml kanamycin, and the OD600 was cultured to OD600 at 37 ° C, 180R / min, and seed bacterial liquid was obtained. The seed bacteria was seeded in a 1% volume concentration to fresh induced medium containing 50 μg / ml kanamycin, which was placed at 30 ° C for 18 h, and the culture solution was obtained. The culture solution was centrifuged at 25 ° C, 8000 R / min, and the supernatant was discarded to collect the precipitate, and the precipitate was cleaned with a pB buffer of 7.0, and the wet bacteria was collected.
[0092] The wet bacteria having a super pure water resuspended is to prepare a bacterial liquid having a concentration of 20%. Ultrasonic crushing or high pressure homogeneous crushing method is treated with bacterial liquid, and the crushing conditions c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com