A class of acetophenone oxime ester imidazole derivatives and preparation method and application thereof
A technology of acetophenone oxime ester imidazole and derivatives is applied in the field of acetophenone oxime ester imidazole derivatives and preparation thereof, and can solve the problems of few activity reports, no activity reports and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
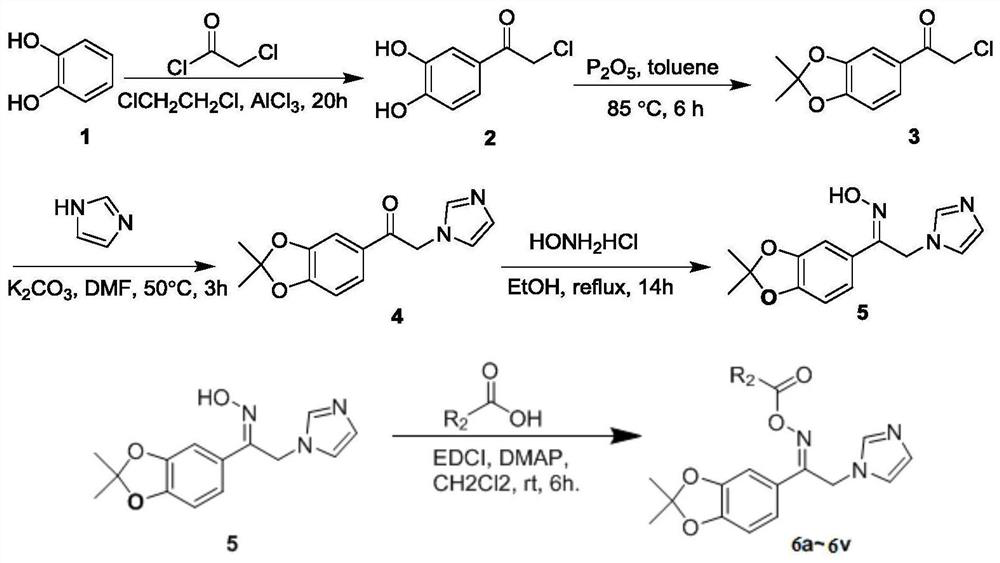
[0033] Preparation of (E)-1-(2,2-dimethylbenzo[d][1,3]dioxol-5-yl)-2-(1H-imidazol-1-yl)ethanone oxime ester method, including the following steps:
[0034] (1) Preparation of 2-chloro-1-(3,4-dihydroxyphenyl)ethanone (compound 2):
[0035] 6.0 g, 45 mmol of aluminum chloride was added to 20 mL of 1,2-dichloroethane, and after stirring at 5 °C for 30 min, 4.0 g, 36.2 mmol of catechol (compound 1) was slowly added under stirring conditions. , and continue to stir for 20 min, then add 4.4 g, 38.6 mmol of chloroacetyl chloride at 5 °C, and naturally warm to room temperature, and then stir at room temperature for 20 h. After the reaction is completed, add 70 mL, 0.5 mol / 1 L of dilute hydrochloric acid solution was quenched, the reaction was stopped at the required stage, then the temperature was naturally raised to room temperature, stirred for 3 h, filtered to obtain a solid and washed with water;
[0036] Decolorize the wet solids, specifically: dissolving the wet solids in an ...
Embodiment 2
[0053] The preparation steps are the same as those in Example 1, except that the carboxylic acid compound is replace with Compound 6b was obtained as colorless oil, yield 48%; 1 H NMR (500MHz, CDCl 3 )δ7.93(d,J=8.0Hz,2H),7.59(s,1H),7.30(d,J=8.0Hz,2H),7.22(s,1H),7.17(d,J=8.0Hz, 1H), 7.06(s, 1H), 6.97(s, 1H), 6.74(d, J=8.1Hz, 1H), 5.36(s, 2H), 2.44(s, 3H), 1.69(s, 6H); 13 C NMR (125MHz, CDCl 3 )δ163.26,159.51,150.54,148.37,144.75,137.40,130.05,129.72,129.56,125.55,125.05,121.81,119.38,119.11,108.37,107.30,42.22,25.87 22 H 21 N 3 O 4 392.1604[M+H] + , found 392.1601.
Embodiment 3
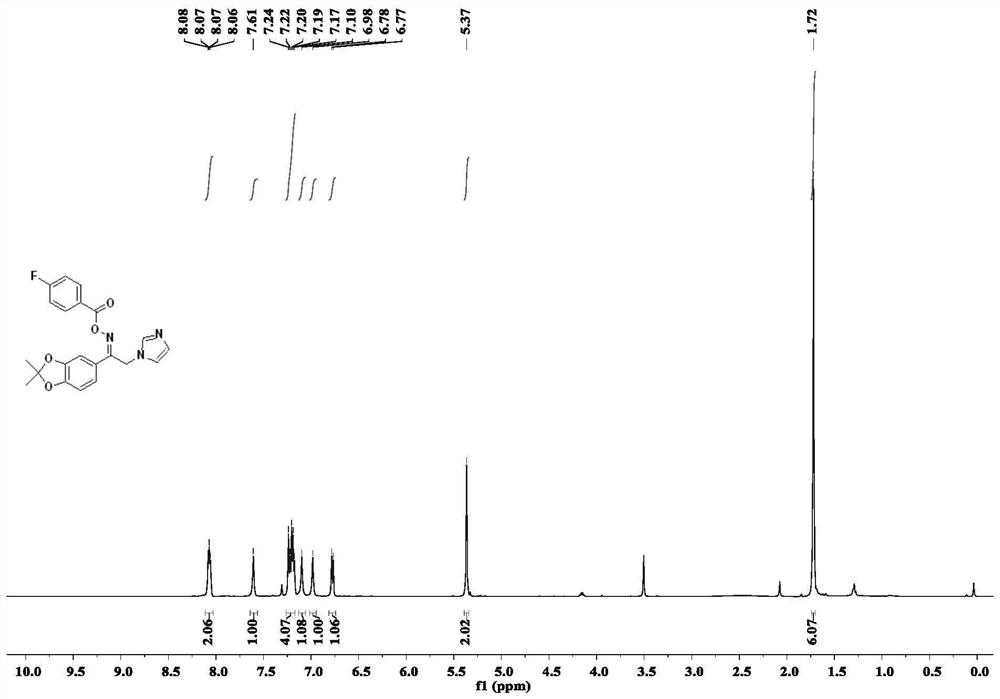
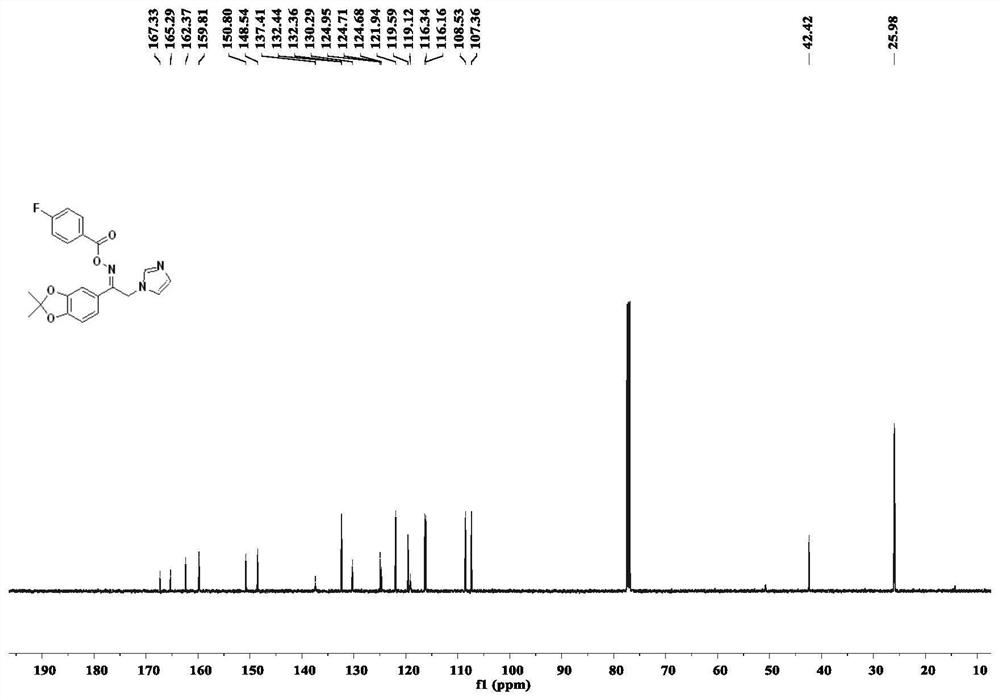
[0055] The preparation steps are the same as those in Example 1, except that the carboxylic acid compound is replace with Compound 6c was obtained as colorless oil, yield 54%; 1 H NMR (500MHz, CDCl 3 )δ8.07(dd,J=7.7Hz,5.7Hz,2H),7.61(s,1H),7.26–7.15(m,4H),7.10(s,1H),6.98(s,1H),6.78( d, J=8.1Hz, 1H), 5.37(s, 2H), 1.72(s, 6H); 13 C NMR (125MHz, CDCl 3 )δ166.31(d,J C-F = 255.8Hz), 162.37, 159.81, 150.80, 148.54, 137.41, 132.40 (d, J C-F = 9.5Hz), 130.29, 124.95, 124.69 (d, J = 3.0 Hz), 121.94, 119.59, 119.12, 116.25 (d, J C-F = 22.2Hz), 108.53, 107.36, 42.42, 25.98; .HRMS(ESI) m / z:calcd for C 21 H 18 FN 3 O 4 396.1354[M+H] + , found 396.1352.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com