Serum-free, protein-free and DMSO-free cell freezing medium and application thereof
A cryopreservation liquid and protein-free technology, which can be used in applications, preservation of human or animal bodies, animal husbandry, etc., can solve the problems of increasing the risk of cell contamination, complicated washing of cells, and the reduction of cell number, so as to maintain high activity And good dryness, safe and reliable clinical application, clear ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
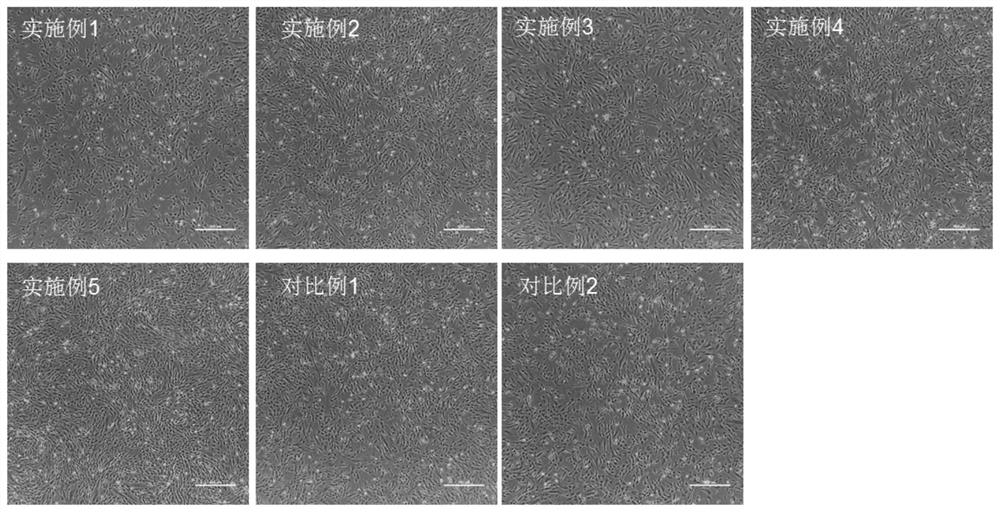
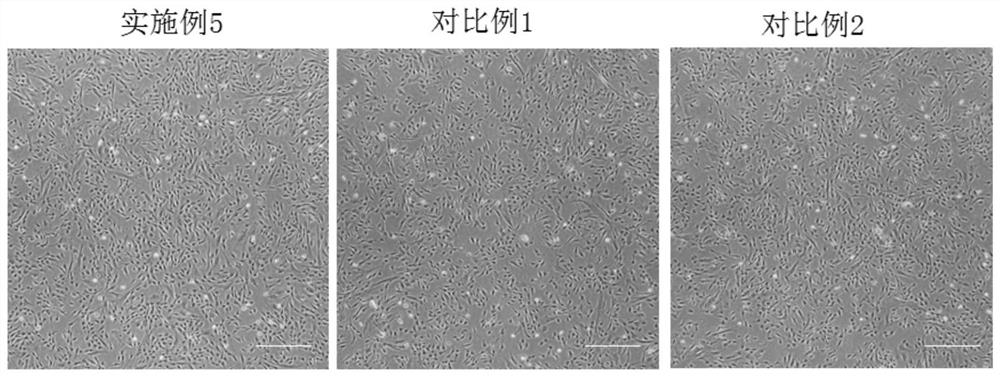
Image
Examples
Embodiment 1
[0099] Embodiment 1 A kind of serum-free, protein-free and DMSO-free cell cryopreservation solution
[0100] A serum-free, protein-free and DMSO-free cell cryopreservation solution, the cell cryopreservation solution is composed of the following components:
[0101]
[0102]
Embodiment 2
[0103] Embodiment 2 A kind of serum-free, protein-free and DMSO-free cell cryopreservation solution
[0104] A serum-free, protein-free and DMSO-free cell cryopreservation solution, the cell cryopreservation solution is composed of the following components:
[0105]
Embodiment 3
[0106] Embodiment 3 A kind of serum-free, protein-free and DMSO-free cell cryopreservation solution
[0107] A serum-free, protein-free and DMSO-free cell cryopreservation solution, the cell cryopreservation solution is composed of the following components:
[0108]
[0109]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com