Biphenyl nitrile derivative with AIE and ESIPT characteristics as well as synthesis method and application of biphenyl nitrile derivative
A synthesis method and technology of biphenylnitrile, applied in chemical instruments and methods, measurement of color/spectral characteristics, and analysis through chemical reaction of materials, etc., can solve problems such as unidentifiable with naked eyes, poor water solubility, and low sensitivity , to achieve clear results, low detection cost, and simple detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
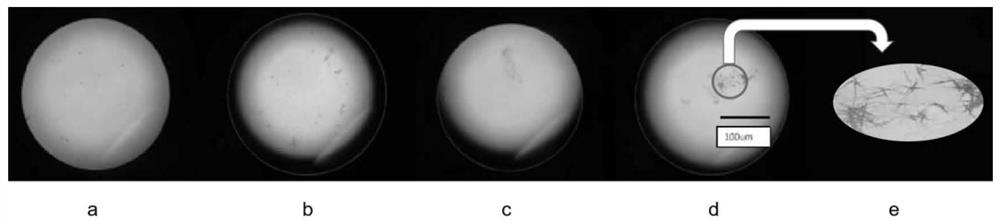
Image
Examples
Embodiment 1
[0035] Synthesis and characterization of embodiment 1 biphenyl nitrile derivative
[0036]Synthesis of 3'-formyl-4'-hydroxyl-4-cyanobiphenyl: According to the literature (A cyanobiphenylcontaining fluorescence "turn on" sensor for Al 3+ ion in CH 3 CN-water, Onder Alici, et al, "Sensors and Actuators B:Chemical", volume 208, pages 159-163) report method to synthesize: 4'-hydroxyl-4-biphenylcarbonitrile (0.5g , 2.56mmol) and hexamethylenetetramine (HMTA, 2.15g, 15.36mmol) in a solution of trifluoroacetic acid (40mL) was refluxed for 4h. After completion, the reaction mixture was cooled to room temperature and 1.0M HCl (100 mL) was added, the resulting mixture was extracted with dichloromethane (100 mL), the organic layer was washed 3 times with water and once with saturated brine, and dried over magnesium sulfate. After filtration, the solvent was removed to give 3'-formyl-4'-hydroxy-4-biphenylcarbonitrile (40.59 mg, 71%) as a yellow solid. 1 H NMR (DMSO-d 6 )δ (ppm): 11.07...
Embodiment 2
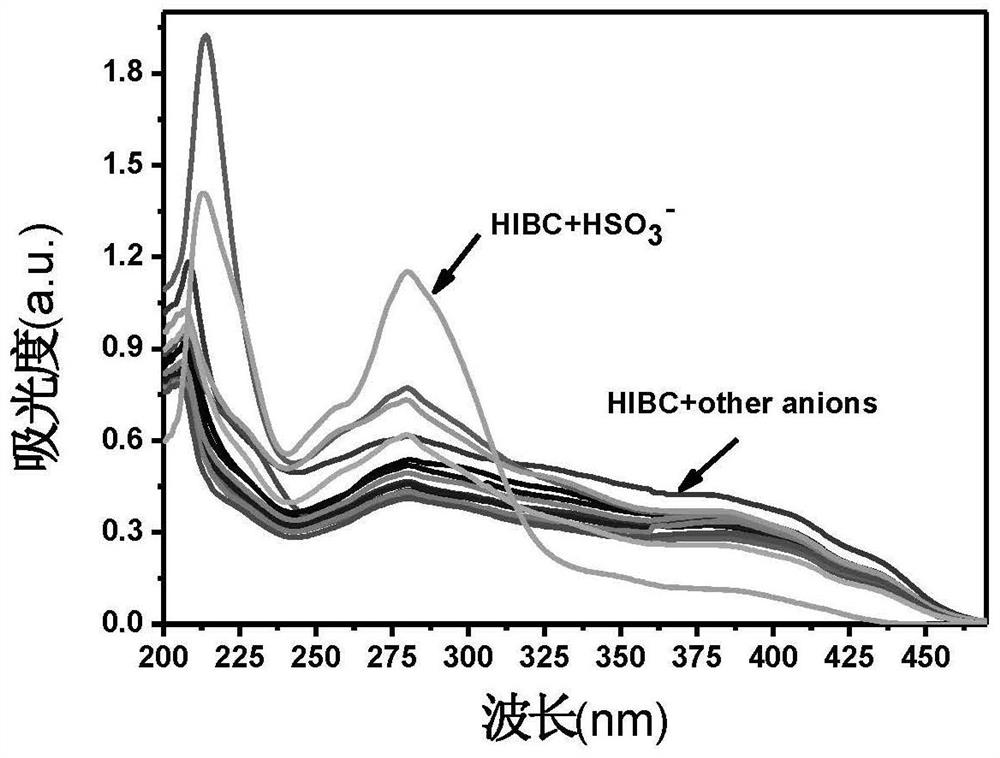
[0038] Embodiment 2 biphenyl nitrile derivatives are used for HSO 3 - UV selectivity determination of
[0039] 1mM NBD derivative stock solution in DMSO, 0.01M HSO in distilled water 3 - Solution, and configure HEPES buffer solution with pH=7.4 and concentration of 0.025M; take 100 μL of biphenyl nitrile derivative stock solution and add it to a clean colorimetric tube, add a certain amount of anion stock solution (F - , Cl - ,Br - ,I - ,AcO - ,CN - ,NO 3 - ,H 2 PO 4 - , HSO 4 - , SO 4 2- , SO 3 2- , HSO 3 - ,S 2- ,CO 3 2- , HCO 3 - ), add 0.5mL HEPES buffer, dilute to 5mL with secondary water, shake well, take 2.5mL into a clean cuvette, and detect it on a UV-Vis spectrophotometer. HIBC itself has two absorption peaks at 275nm and 395nm. After adding common anions, only HSO 3 - Reduce the absorption peak of HIBC at 395nm, and enhance the absorption peak at 275nm, indicating that HIBC has the effect on HSO 3 - There is good selectivity. UV select...
Embodiment 3
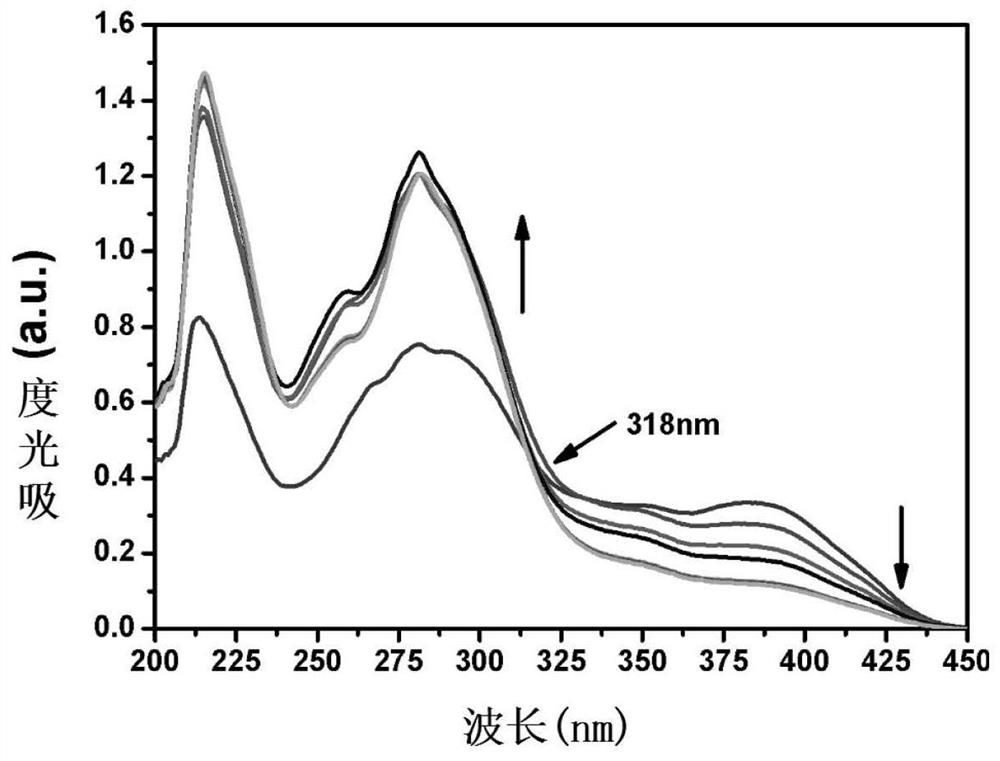
[0040] Embodiment 3 biphenyl nitrile derivatives are used for HSO 3 - UV absorbance determination
[0041] Take 100 μL of biphenyl nitrile derivative stock solution and add it to a clean colorimetric tube, add different volumes of HSO 3 - (2μL, 4μL, 6μL, 8μL, 10μL, 12μL, 14μL, 16μL), add 0.5mL HEPES buffer, dilute to 5mL with secondary water, shake well and add 2.5mL to a clean cuvette, visible in UV detected on a spectrophotometer, with HSO 3 - The addition of the solution gradually changed from yellow to colorless, the absorption peak at 395nm gradually decreased, the absorption peak at 275nm gradually increased, and the isosbestic point appeared at 318nm. The UV spectrum absorption diagram is shown in image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com