Separation and detection method of dexrazodone intermediate and impurities
A detection method, the technology of dexrazoxane, is used in material separation, measurement devices, analysis materials, etc., and can solve problems such as unfavorable daily operations, small number of separated impurities, and low column temperature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] Preparation of sample solution: Take 10 mg of dexrazoxane containing impurities, 5 mg of intermediate M2 and intermediate M1, dissolve and dilute with phosphoric acid solution with a volume fraction of 1.0%, precisely dilute to a 10.0 mL volumetric flask, shake well, Place in a water bath at 80°C for 3 hours to obtain the sample solution.
[0046] High performance liquid chromatography detection conditions:
[0047] High performance liquid chromatography: Thermo Fisher U3000, DAD ultraviolet detector;
[0048] Chromatographic column: CAPCELL PAK ADME chromatographic column, 4.6mm×250mm, 5um;
[0049] Mobile phase: A: 1% methanol-0.1% phosphoric acid; B: 40% acetonitrile-0.1% phosphoric acid;
[0050] Injection volume: 10μL;
[0051] Running time: 50min;
[0052] Detection wavelength: 210nm;
[0053] Gradient elution: The elution program is shown in Table 1:
[0054] Table 1 Gradient elution program (volume fraction)
[0055]
[0056]
Embodiment 1~3
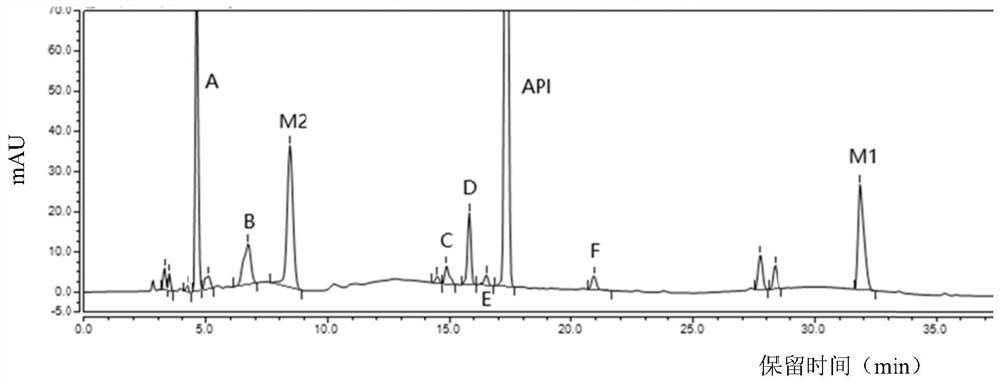
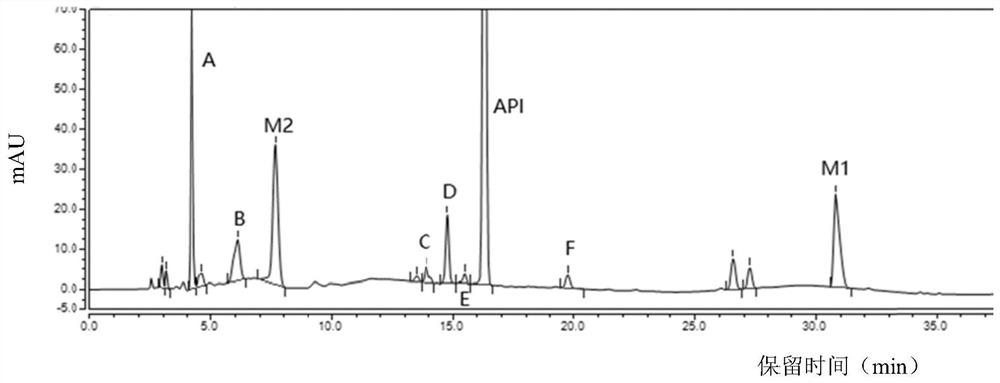
[0057] Embodiments 1 to 3: When the column temperature of the chromatographic column is 25°C, the flow velocity of the mobile phase is respectively 0.9mL / min, 1.0mL / min, and 1.1mL / min to detect the sample solution, and record the chromatograms to obtain Figure 1~3 , the retention time and resolution of each peak are shown in Table 2:
[0058] Retention time and resolution of each peak under different flow rates of mobile phase in table 2
[0059]
[0060] As can be seen from Table 2, in Examples 1 to 3, after the flow velocity of the mobile phase of the chromatographic column changes, the retention time of each peak changes accordingly, but within this flow velocity range, dexrazoxane, intermediates M2, M1 and impurity A The separation of impurity F is good, and the overall effect is the best when the mobile phase flow rate is 1.0mL / min, which shows that the method can well control the quality of dexrazoxane samples.
Embodiment 4~6
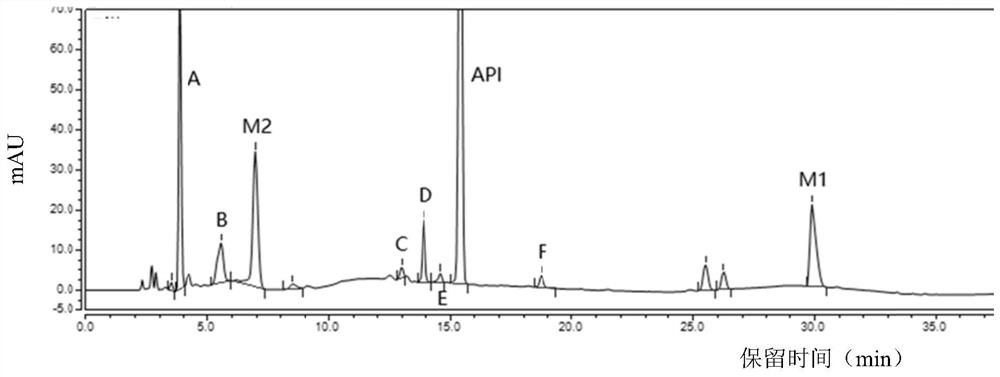
[0061] Embodiments 4-6: When the flow rate of the mobile phase is 1.0mL / min, the column temperature of the chromatographic column is respectively set to 20°C, 25°C, and 30°C, the sample solution is detected, and the chromatograms are recorded in order to obtain Figure 4~6, the retention time and resolution of each peak are shown in Table 3:
[0062] Table 3 Retention time and resolution of each peak at different column temperatures
[0063]
[0064] As can be seen from Table 3, in Examples 4-6, after the column temperature of the chromatographic column changes, the retention time of each peak changes accordingly, but within this column temperature range, dexrazoxane, intermediates M2, M1 and impurities The separation of A to impurity F is good, and the overall effect is the best when the column temperature is 25°C, which shows that the method can well control the quality of dexrazoxane samples.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com