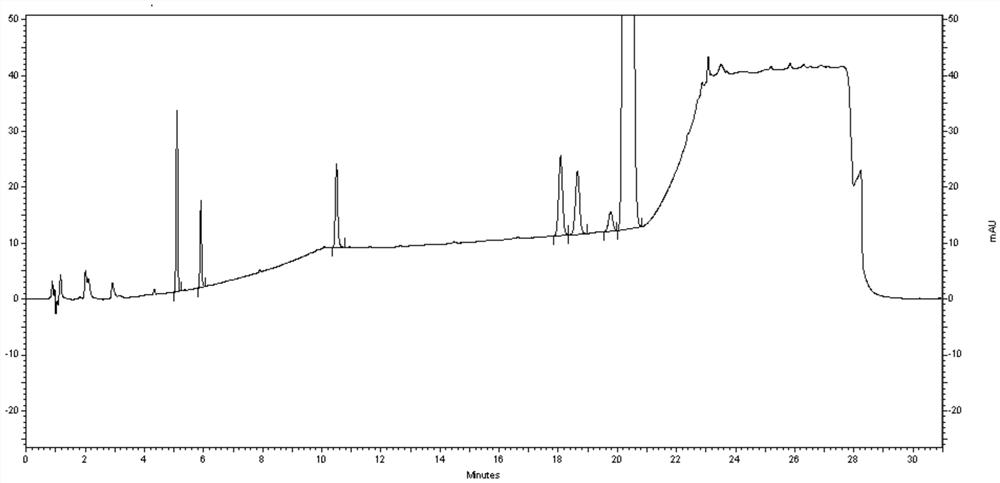
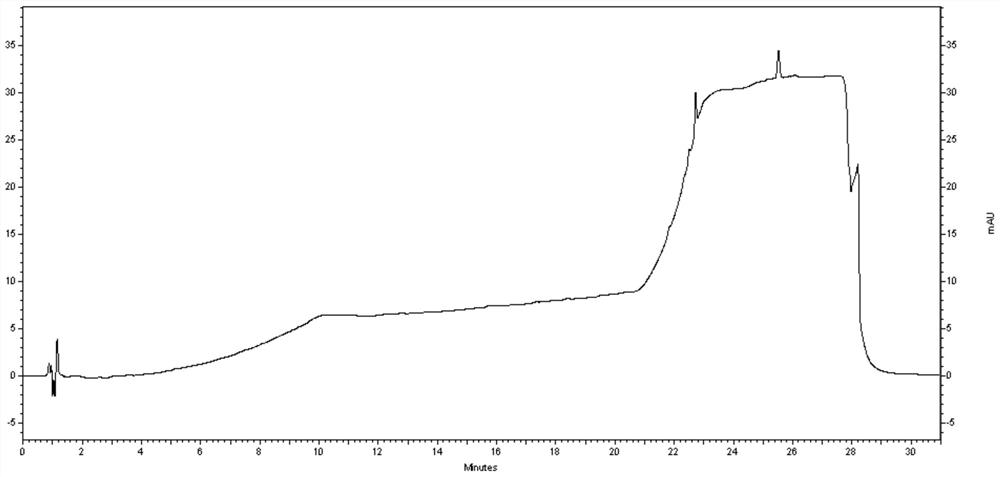
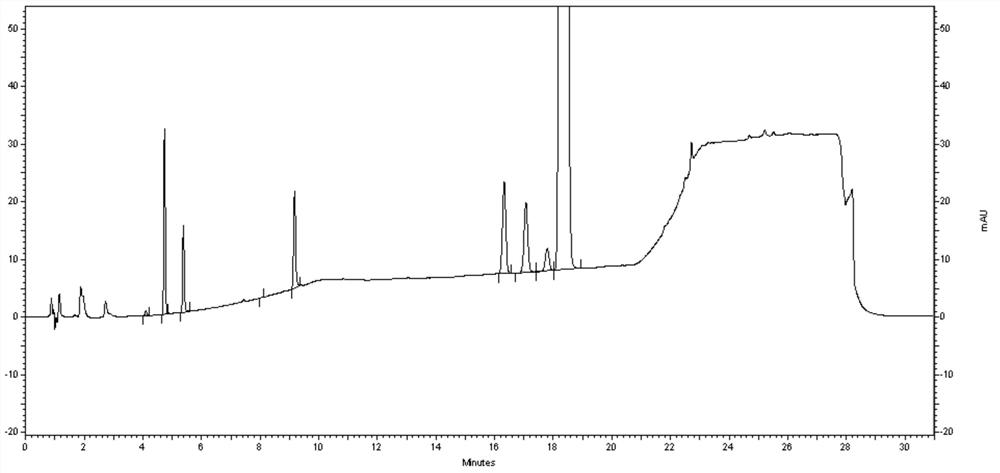
Method for determining efinaconazole related substances by HPLC (High Performance Liquid Chromatography) method
A technology related to substances, potassium dihydrogen phosphate, applied in the field of analytical chemistry, can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072]Instruments and chromatography conditions:
[0073]Chromatographic system: USA Agilent 1260 high-performance liquid chromatography system and workstation;
[0074]Columns: Mn Nucleshell RP18-Plus, 4.6x100mm, 2.7 μm;
[0075]Detector: UV detector;
[0076]Test wavelength: 210 nm;
[0077]Mobile phase a phase: 1.36 g / L of pelicate solution, adjusting pH to 6.5 with phosphoric acid, wherein the volume ratio of pelicine dihydrogen phosphate solution and acetonitrile is 95: 5;
[0078]Mobile phase B phase: mixed solvent of acetonitrile with methanol, wherein the volume ratio of acetonitrile to methanol is 3: 1;
[0079]The gradient elution procedure is as follows;
[0080]
[0081]Chromatography column temperature: 30 ° C;
[0082]Flow rate: 1.0ml / min;
[0083]Number of injections: 10 μL.
[0084]Experimental steps:
[0085]Diluent / Blank Solvent: Acetonitrile: Water = 50: 50 (V / V);
[0086]Try to test the sample: Take about 25 mg of Aiflufluornazole to the test product, precisely weighted to 25 ml of brown volumetr...
Embodiment 2
[0090]Instruments and chromatography conditions:
[0091]Chromatographic system: USA Agilent 1260 high-performance liquid chromatography system and workstation;
[0092]Columns: Mn Nucleshell RP18-Plus, 4.6x100mm, 2.7 μm;
[0093]Detector: UV detector;
[0094]Test wavelength: 210 nm;
[0095]Mobile phase a phase: 1.36 g / L of pelicate solution, adjusting pH to 6.5 with phosphoric acid, wherein the volume ratio of pelicine dihydrogen phosphate solution and acetonitrile is 95: 5;
[0096]Mobile phase B phase: mixed solvent of acetonitrile with methanol, wherein the volume ratio of acetonitrile to methanol is 3: 1;
[0097]The gradient elution procedure is as follows;
[0098]
[0099]Chromatography column temperature: 20 ° C;
[0100]Flow rate: 1.0ml / min;
[0101]Number of injections: 10 μL.
[0102]Experimental steps:
[0103]Diluent / Blank Solvent: Acetonitrile: Water = 50: 50 (V / V);
[0104]Try to test the sample: Take about 25 mg of Aiflufluornazole to the test product, precisely weighted to 25 ml of brown volumetr...
Embodiment 3
[0108]Instruments and chromatography conditions:
[0109]Chromatographic system: USA Agilent 1260 high-performance liquid chromatography system and workstation;
[0110]Columns: Mn Nucleshell RP18-Plus, 4.6x100mm, 2.7 μm;
[0111]Detector: UV detector;
[0112]Test wavelength: 210 nm;
[0113]Mobile phase a phase: 1.36 g / L of pelicate solution, adjusting pH to 6.5 with phosphoric acid, wherein the volume ratio of pelicine dihydrogen phosphate solution and acetonitrile is 95: 5;
[0114]Mobile phase B phase: mixed solvent of acetonitrile with methanol, wherein the volume ratio of acetonitrile to methanol is 3: 1;
[0115]The gradient elution procedure is as follows;
[0116]
[0117]Chromatography column temperature: 15 ° C;
[0118]Flow rate: 1.0ml / min;
[0119]Number of injections: 10 μL.
[0120]Experimental steps:
[0121]Diluent / Blank Solvent: Acetonitrile: Water = 50: 50 (V / V);
[0122]Try to test the sample: Take about 25 mg of Aiflufluornazole to the test product, precisely weighted to 25 ml of brown volumetr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com