Synthesis method of N-aryl amide with recoverable raw materials
The technology of an arylamide and a synthesis method is applied in the field of N-arylamide synthesis with recyclable raw materials, and can solve the problems of high cost of catalyst raw materials, long reaction time, low atom utilization rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
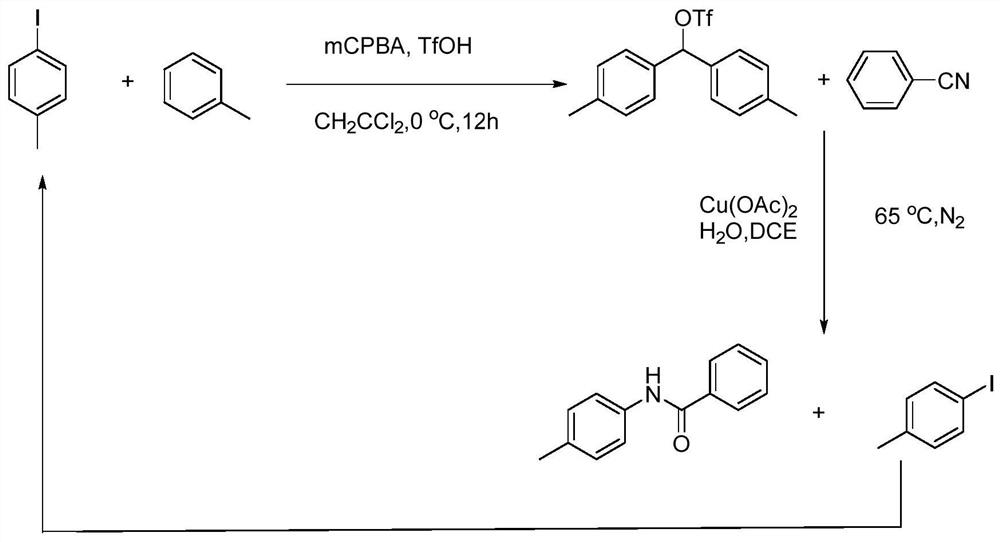
[0043] figure 1 A schematic diagram of a recyclable N-arylamide synthetic method is shown, and the specific process is:
[0044] S1: Add 2.4mmol of p-methyliodobenzene, 2.76mmol of mCPBA, 2.76mmol of toluene, and 17mL of dichloromethane into the vacuum reaction flask, then put it in an ice bath at 0°C, and use a rubber-tipped dropper to drop Add 0.6 mL of trifluoromethanesulfonic acid, and stir at room temperature for 12 hours after two hours;
[0045] S2: Continue to add about 5.7mg of Cu(OAc) to S1 2 , 2 mL of DCE, 0.7 mmol of H 2 O and 1.0mmol of benzonitrile are then connected to the vacuum / inert gas manifold system and fed with nitrogen for protection, heated in an oil bath, and the temperature is kept at 80°C, and the reaction is continuously stirred for 20 hours;
[0046] S3: After the reaction is complete, cool to room temperature, filter, and filter out Cu(OAc) 2 , EtOAc was added to the filtrate, stirred for half an hour, and the organic layer was separated. Was...
Embodiment 2
[0050] figure 2 A schematic diagram of a recyclable N-arylamide synthetic method is shown, and the specific process is:
[0051] S1: Add 2.4mmol of 3,4-dimethyliodobenzene, 2.76mmol of mCPBA, 2.76mmol of o-xylene, and 17mL of dichloromethane into a vacuum reaction flask, then put it in an ice bath at 0°C, and use Add 0.6 mL of trifluoromethanesulfonic acid drop by drop with a rubber dropper, and stir at room temperature for 12 hours after two hours;
[0052] S2: Add about 5.7 mg of CuI, 2 mL of DCE, and 0.7 mmol of H to S1 2 O, 1.0 mmol of benzonitrile was connected to the vacuum / inert gas manifold system to feed nitrogen for protection, heating, the temperature was maintained at 75 ° C, and the reaction was continued for 17 hours with stirring;
[0053] S3: The reaction is completed, cooled to room temperature, filtered, and Cu(OAc)2 was filtered out, EtOAc was added to the filtrate, stirred for half an hour, and the organic layer was separated. Wash the organic layer wit...
Embodiment 3
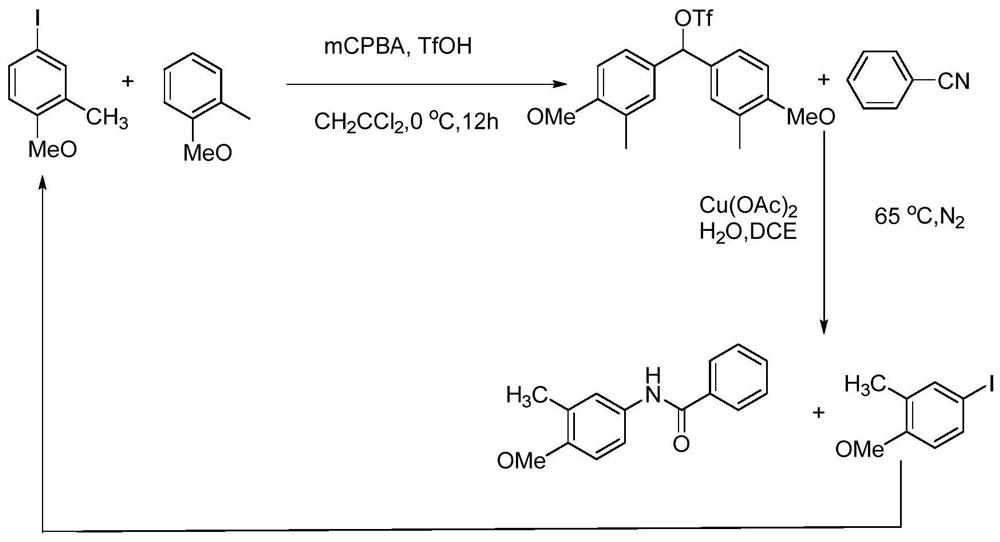
[0057] image 3 A schematic diagram of a recyclable N-arylamide synthetic method is shown, and the specific process is:
[0058] S1: Add 2.4mmol of 4-methoxy-3-methylbenzene, 2.86mmol of mCPBA, 2.76mmol of 2-methylanisole, and 20mL of dichloromethane into a vacuum reaction flask, and then put In an ice bath, add 0.75 mL of trifluoromethanesulfonic acid dropwise using a rubber dropper, and stir at room temperature for 19 hours after two hours;
[0059] S2: Continue to add about 5.7mg of CuI, 2mL of DCE, 0.7mmol of H to S1 2 O, 1.0 mmol of benzonitrile was then connected to the vacuum / inert gas manifold system and fed with nitrogen for protection, heating, the temperature was maintained at 75 ° C, and the reaction was continued for 18 hours with stirring;
[0060] S3: After the reaction is complete, cool to room temperature, filter, and filter out Cu(OAc) 2 , EtOAc was added to the filtrate, stirred for half an hour, and the organic layer was separated. Wash the organic laye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com