Preparation method of N, N-dimethylacetamide
A technology of dimethylacetamide and methylpyrimidine, which is applied in the field of preparation of 2--N,N-dimethylacetamide, can solve the problems of complicated post-treatment process, poor atom economy and high reaction cost, and achieve the goal of reaction And the effect of simple post-processing, low cost and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a preparation method of 2-(2-n-butyl-4-hydroxyl-6-methylpyrimidin-5-yl)-N,N-dimethylacetamide, comprising the following steps:
[0030] 1) Make pentamidine hydrochloride and dimethyl acetylsuccinate react under the effect of organic amine to obtain 2-(2-n-butyl-4-hydroxyl-6-methylpyrimidin-5-yl)-acetic acid methyl Esters, wherein the pKa of the organic amine is 8-11.5;
[0031] 2) Reaction of 2-(2-n-butyl-4-hydroxyl-6-methylpyrimidin-5-yl)-acetic acid methyl ester with dimethylamine to obtain 2-(2-n-butyl-4-hydroxyl -6-methylpyrimidin-5-yl)-N,N-dimethylacetamide.
[0032] Above-mentioned preparation method can be represented by following chemical reaction formula:
[0033]
[0034] In the reaction of pentamidine hydrochloride and dimethyl acetylsuccinate, it is necessary to use alkali to neutralize the HCl in pentamidine hydrochloride, so that the nitrogen atom in the carbon-nitrogen double bond in pentamidine hydrochloride It has nucleophil...
Embodiment 1
[0052] The preparation method of the present embodiment 2-(2-n-butyl-4-hydroxyl-6-methylpyrimidin-5-yl)-N,N-dimethylacetamide is as follows:
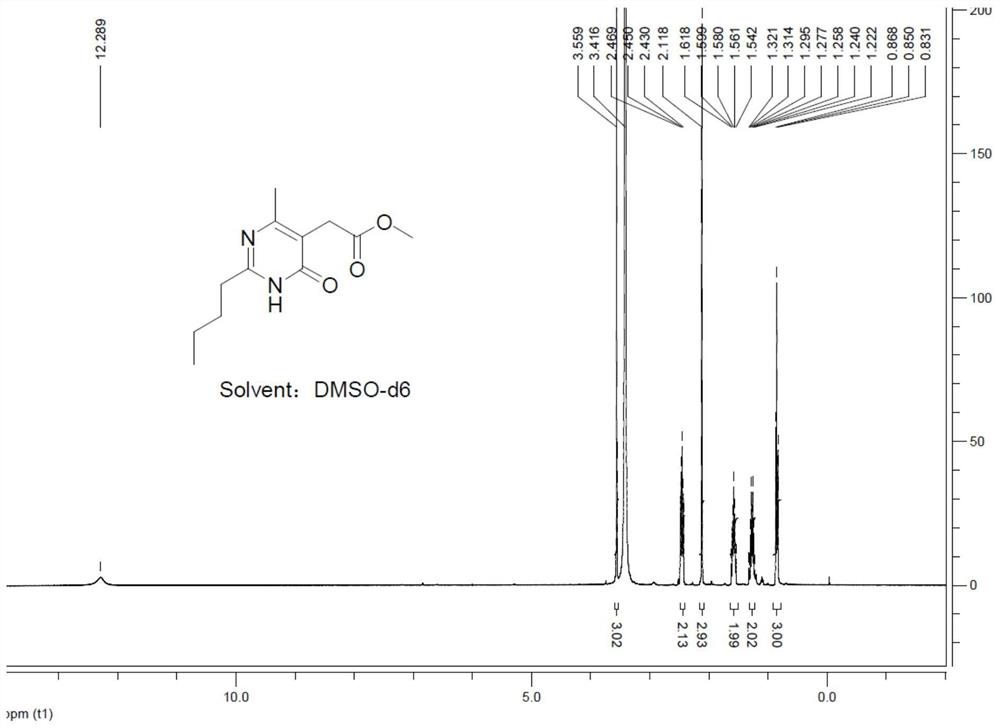
[0053] 1) Preparation of 2-(2-n-butyl-4-hydroxyl-6-methylpyrimidin-5-yl)-acetic acid methyl ester
[0054] At room temperature, 13.7 g (0.1 mol) of pentamidine hydrochloride and 20.7 g (0.11 mol, 1.1 equiv) of dimethyl acetylsuccinate were added to a 250 mL four-necked flask, and 70 mL of methanol was added to dissolve the reactant completely. Under stirring state, use the constant pressure dropping funnel to add dropwise N,N-diisopropylethylamine 25.8g (0.2mol, 2.0equiv, pKa value is 10.98), control dropping time to be 0.5h, dropwise add The system was heated to reflux for 4 hours.
[0055] After cooling the reaction system to room temperature, concentrate under reduced pressure to remove methanol to obtain a concentrate, add 100 mL of water to the concentrate, add 13 g of concentrated hydrochloric acid under stirring to adjust the pH...
Embodiment 2
[0063] The preparation method of the present embodiment 2-(2-n-butyl-4-hydroxyl-6-methylpyrimidin-5-yl)-N,N-dimethylacetamide is as follows:
[0064] 1) Preparation of 2-(2-n-butyl-4-hydroxyl-6-methylpyrimidin-5-yl)-acetic acid methyl ester
[0065] At room temperature, add 27.4g (0.2mol) of pentamidine hydrochloride and 41.4g (0.22mol, 1.1equiv) of dimethyl acetylsuccinate into a 500mL four-neck flask, add 70mL of methanol to completely dissolve the reactants, and stir 51.6g (0.4mol, 2.0equiv) of N, N-diisopropylethylamine was added dropwise using a constant pressure dropping funnel, and the dropping time was controlled to be 1h. After the dropping was completed, the system was heated to reflux for 4 hours .
[0066] After cooling the reaction system to room temperature, concentrate under reduced pressure to remove methanol to obtain a concentrate, add 200 mL of water to the concentrate, add 26 g of concentrated hydrochloric acid under stirring to adjust the pH to 3, and use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com