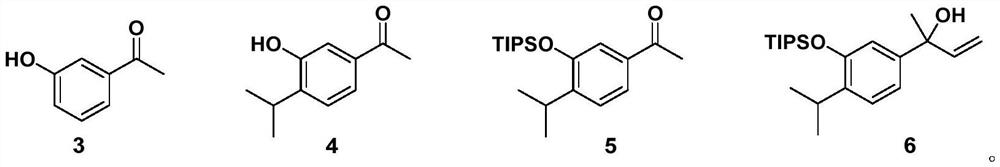
Method for asymmetrically synthesizing Triptonide and Triptolide
An asymmetric and unsaturated technology, applied in asymmetric synthesis, chemical instruments and methods, organic chemistry methods, etc., can solve the problems of low synthesis efficiency and long steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0061] The present invention will be described in further detail below by way of examples.
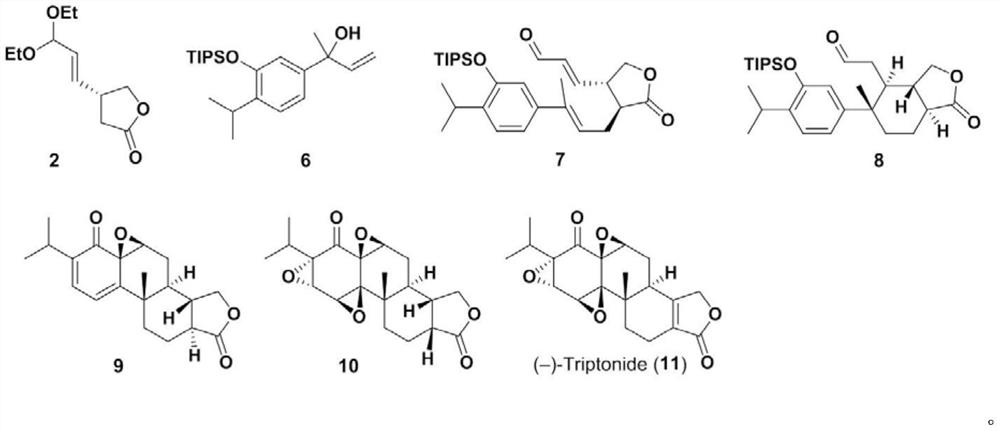
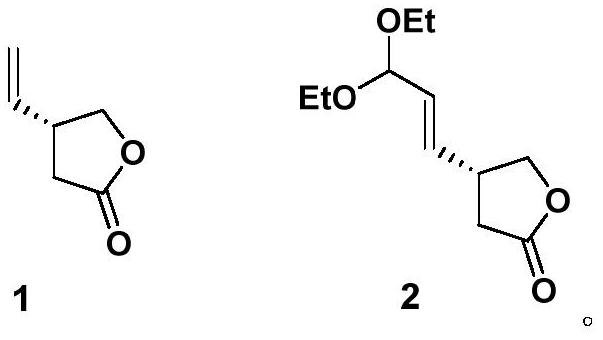
[0062] Synthesis of compound 2:
[0063]
[0064] To the CH of compound 1 (2g, 17.9mmol) 2 Cl 2 (88 mL) was added S1 (5.4 mL, 35.7 mmol) followed by Hoveyda-Grubbs second generation catalyst (276 mg, 0.45 mmol). Connect the flask to a condenser, replace the nitrogen, and then place the reaction system in an oil bath at 45°C. After 4 hours, add Hoveyda-Grubbs second-generation catalyst (276mg, 0.45mmol) and S1 (5.4mL, 35.7mmol) to continue After reacting at 45°C for 12 hours, the reaction system was cooled to room temperature. The solvent was spun off in vacuo, and the residue was purified by column chromatography (silica gel, petroleum ether / ethyl acetate=10:1 to 5:1) to obtain compound 2, a colorless oil, 2.6 g, 12.1 mmol, yield 68%.
[0065] The detection data of compound 2 are as follows:
[0066] R f =0.31 (petroleum ether / ethyl acetate=2:1)
[0067]
[0068] HRMS-ESI(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com