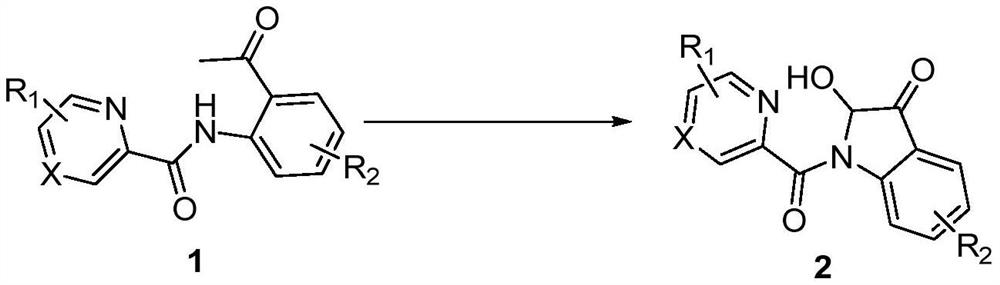
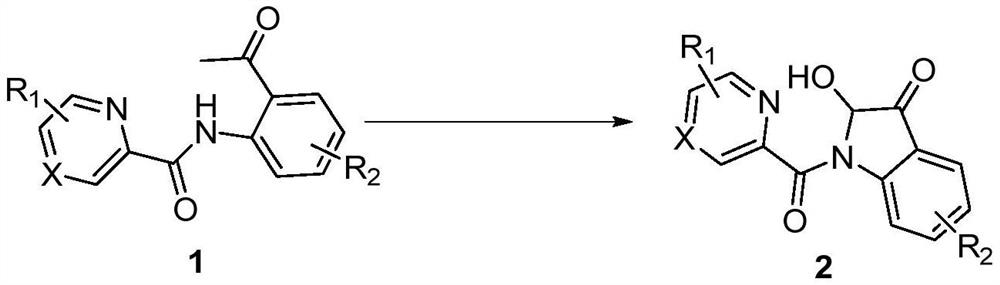
Synthesis method of 2-hydroxy-indole-3-ketone compound
A technology of ketone compounds and synthetic methods, which is applied in the direction of organic chemistry, can solve the problems of low yield, and achieve the effects of high yield, cost saving, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016]
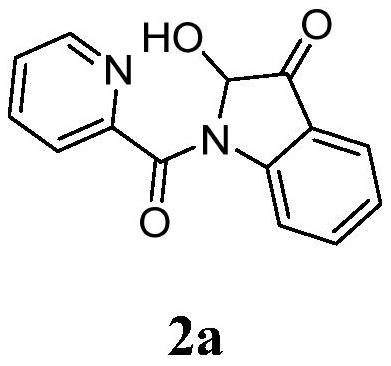
[0017] In a 10mL reaction tube, add 48.0mg (0.2mmol) N-(2-acetylphenyl) pyridine amide, 11.4mg (0.06mmol) cuprous iodide, 24.0mg (0.4mmol) acetic acid, 1.5mL dimethyl sulfoxide, the reaction tube was evacuated, magnetically stirred, and reacted at 100°C for 5 hours in an oxygen atmosphere. TLC monitors that the reaction is complete, wait for the reaction solution to cool to room temperature, extract 3 times with ethyl acetate, wash the organic phase with saturated saline solution, then dry with anhydrous sodium sulfate, concentrate by distillation under reduced pressure, and separate by column chromatography (the eluent is Petroleum ether and ethyl acetate volume ratio 5:1 mixed liquor), obtain target product 2a, chemical name is 2-hydroxyl-indol-3-one, and its yield is 77%, and structural characterization data is as follows:
[0018] 1 H NMR (400MHz, Chloroform-d) δ8.70–8.61(m,2H),8.40(s,1H),8.30(dt,J=8.0,1.1Hz,1H),8.04(td,J=7.8,1.8 Hz,1H),7.88–7.82(m,1H),7.79–7...
Embodiment 2
[0020]
[0021] In the present embodiment, replace the N-(2-acetylphenyl) pyridine amide used in embodiment 1 with the N-(2-acetyl group-6-methylphenyl) pyridine carboxylic acid amide of equimolar amount, other The steps were the same as in Example 1 to obtain the target product 2b with a yield of 60%, and the structural characterization data are as follows:
[0022] 1 H NMR (400MHz, Chloroform-d) δ8.70(d, J=4.1Hz, 1H), 8.33(d, J=7.9Hz, 1H), 8.02(td, J=7.8, 1.8Hz, 1H), 7.68 (d,J=7.0Hz,1H),7.65–7.60(m,1H),7.58(d,J=7.5Hz,1H),7.34–7.24(m,2H),5.43(s,1H),2.35( s,3H); 13 C NMR (100MHz, Chloroform-d) δ195.20, 164.88, 152.27, 151.15, 148.02, 139.84, 138.49, 130.77, 127.42, 126.36, 126.19, 125.70, 122.25, 83.12, 20.76; HRMS C 15 h 13 N 2 o 3 [M+H] + , The theoretical value is 269.0921, and the measured value is 269.0922.
Embodiment 3
[0024]
[0025] In the present embodiment, replace the N-(2-acetylphenyl) pyridine amide used in embodiment 1 with N-(2-acetyl-5-methylphenyl) pyridine carboxylic acid amide of equimolar amount, other The steps were the same as in Example 1 to obtain the target product 2c with a yield of 76%, and the structural characterization data are as follows:
[0026] 1 H NMR (400MHz, Chloroform-d) δ8.65 (d, J = 4.5Hz, 1H), 8.47 (s, 1H), 8.38–8.25 (m, 2H), 8.03 (td, J = 7.8, 1.8Hz, 1H), 7.72(d, J=7.8Hz, 1H), 7.64–7.58(m, 1H), 7.13(d, J=7.8Hz, 1H), 5.57(s, 1H), 2.52(s, 3H); 13 C NMR (100MHz, Chloroform-d) δ193.92, 164.45, 153.13, 151.90, 149.65, 147.26, 138.80, 126.97, 126.67, 124.41, 121.04, 119.76, 82.04, 22.84; HRMS C 15 h 13 N 2 o 3 [M+H] + , The theoretical value is 269.0921, and the measured value is 269.0919.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com