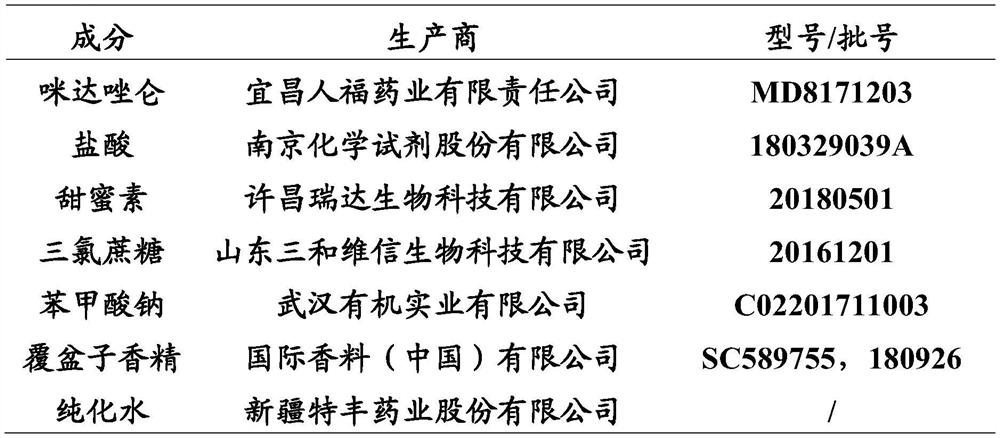
Midazolam liquid preparation, preparation method therefor and use of midazolam liquid preparation
A technology of liquid preparations and midazolam, which is applied in the field of pharmaceutical preparations, can solve the problems of sodium saccharin carcinogenicity and poor stability of syrups, so as to improve product safety, meet children's clinical drug needs, and reduce degradation products. The effect produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0091] Experimental Example 1 Stability Test of Influencing Factors
[0092] With the midazolam oral solution that U.S. commercially available midazolam syrup and the general preparation method of the present invention make, according to Chinese Pharmacopoeia 2020 edition four general rule 9001 regulations, respectively put high temperature (60 ℃ ± 2 ℃), light (4500lx ±500lx, 25°C) to carry out the influence factor test, take samples at 0 and 30 days respectively, investigate related substances and contents, and evaluate palatability.
[0093] Table 4 Influencing factor experiment
[0094]
[0095] Note: / No relevant information. * The palatability is comprehensively evaluated from three aspects: smell, taste and aftertaste. The total score of 5 people is 45 points. The higher the score, the better the taste.
[0096] Compared with the midazolam syrup commercially available in the United States, the number of degradation products, the maximum single impurity, and the tot...
experiment example 2
[0097] Experimental Example 2 Accelerated Stability Test
[0098] With midazolam oral solution that U.S. commercially available midazolam syrup and the embodiment of the present invention make, according to Chinese Pharmacopoeia 2015 edition four general rule 9001 regulations, carry out accelerated stability test, respectively at 0,1,2,3 , June (reference preparation 0, March, and June) samples were taken, the pH value, related substances and contents were investigated, and the palatability was evaluated.
[0099] Table 5 accelerated stability test
[0100]
[0101] Note: * The palatability is comprehensively evaluated from three aspects: smell, taste and aftertaste. The total score of 5 people is 45 points. The higher the score, the better the taste.
[0102] Accelerated for 6 months, the content of midazolam syrup commercially available in the U.S. was significantly reduced to 88.9%, and the total impurities reached 9.3%. 0.30%, the stability is significantly better th...
experiment example 3
[0103] Experimental example 3 long-term stability test
[0104]Put the midazolam syrup commercially available in the United States and the midazolam oral solution prepared in the embodiment of the present invention at 25 ± 2°C, RH 60% The long-term stability test was carried out under the condition of ±5%, and samples were taken at 0, 3, 6, 9, 12, 18, 24, and 36 months, respectively, and the pH, degradation products and content were detected to evaluate the palatability.
[0105] Table 6 Long-term stability experiment
[0106]
[0107] Note: * The palatability is comprehensively evaluated from three aspects: smell, taste and aftertaste. The total score of 5 people is 45 points. The higher the score, the better the taste.
[0108] Compared with the 18-month storage validity period of the commercially available midazolam syrup in the U.S., the midazolam oral solution provided by the present invention has a storage validity period of up to 36 months at room temperature, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com