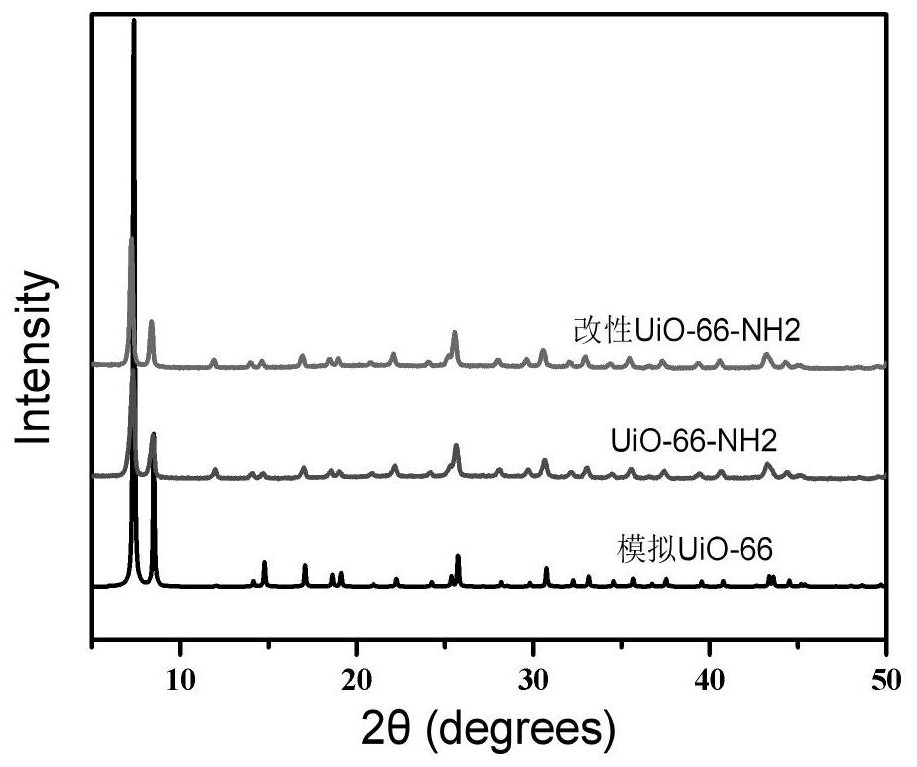
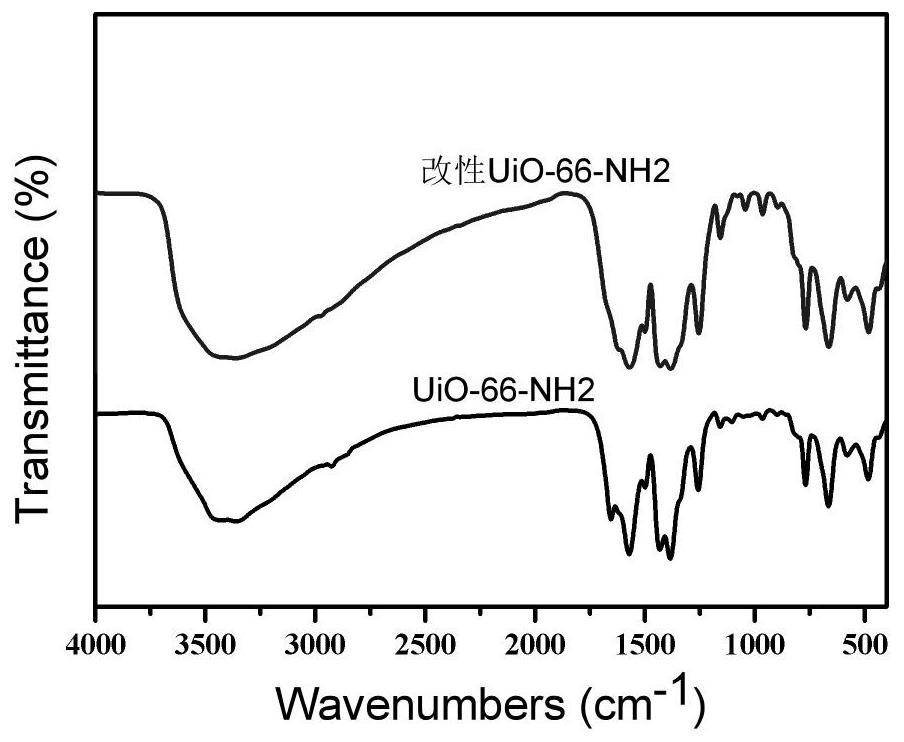
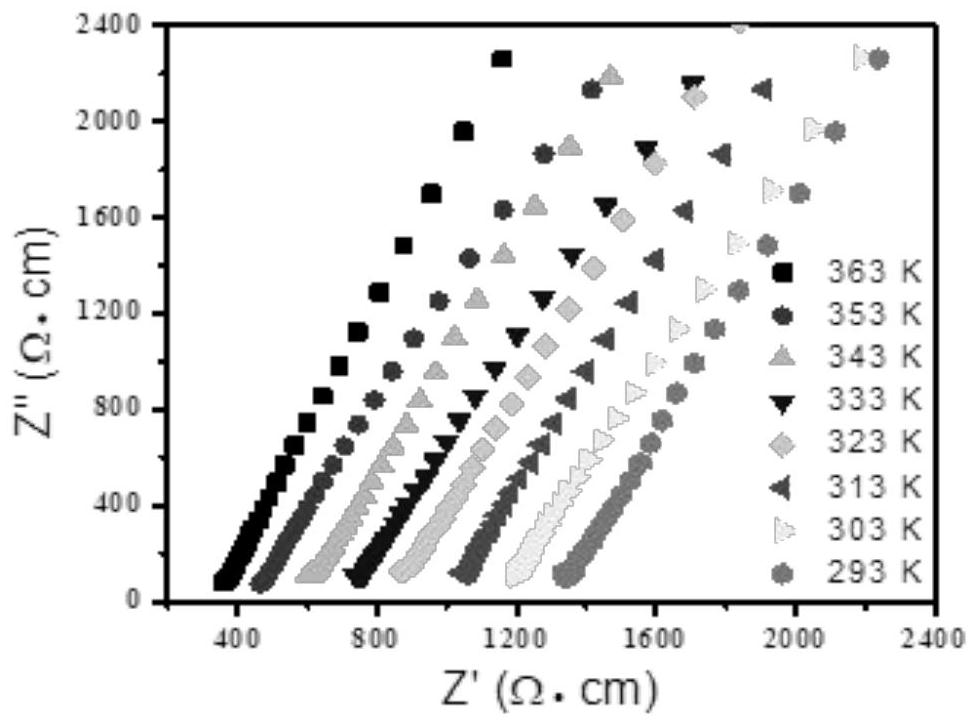
Application of modified UiO-66-NH2 material as proton conduction material
A uio-66-nh2, proton conduction technology, used in the analysis of materials, material analysis by electromagnetic means, fuel cells, etc., to achieve high conductivity value and improve the effect of proton conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) UiO-66-NH 2 Preparation of:
[0034] Add 20g of zirconium tetrachloride and 40g of 2-aminoterephthalic acid into 200mL of N,N-dimethylformamide, and add 5mL of 0.6mol / L hydrochloric acid, react at 80°C for 6h, and obtain a light yellow powder The solid was washed three times with N,N-dimethylformamide, collected and dried under vacuum at 60°C to obtain UiO-66-NH 2 ;
[0035] (2) UiO-66-NH 2 modification of:
[0036] UiO-66-NH 2 5g, 2.5g of isophthalaldehyde and 2.5g of 3,5-diamino-1,2,4-triazole were added to 50mL of N,N-dimethylformamide, and reacted at 25°C for 2h to obtain The dark yellow powder solid was washed three times with N,N-dimethylformamide, collected and dried under vacuum at 40°C to obtain the modified UiO-66-NH 2 .
Embodiment 2
[0038] UiO-66-NH 2 Prepare with embodiment 1;
[0039] UiO-66-NH 2 5g, 2.5g of isophthalaldehyde and 5g of 3,5-diamino-1,2,4-triazole were added to 50mL of N,N-dimethylformamide, and reacted at 25°C for 2h, the obtained deep The yellow powder solid was washed three times with N,N-dimethylformamide, collected and dried under vacuum at 40°C to obtain the modified UiO-66-NH 2 .
Embodiment 3
[0041] UiO-66-NH 2 Prepare with embodiment 1;
[0042] UiO-66-NH 2 5g, 5g of isophthalaldehyde and 2.5g of 3,5-diamino-1,2,4-triazole were added to 50mL of N,N-dimethylformamide, and reacted at 25°C for 2h, the obtained deep The yellow powder solid was washed three times with N,N-dimethylformamide, collected and dried under vacuum at 40°C to obtain the modified UiO-66-NH 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com