Quinoxaline derivative polymer material based on side chain halogen atom substitution and application
A technology of polymer materials and halogen atoms, which is applied in the field of polymer solar cells, can solve the problems of high-efficiency devices such as complicated process and few types, and achieve the effect of promoting electron transmission, reducing influence, and increasing open circuit voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The following is the synthesis of polymers PBDTS-DFQ and PBDTS-DClQx, and its synthetic route is as follows:
[0036]
[0037] Synthesis of compound M1
[0038] Dissolve 5-bromo-2-fluorophenol (1.91g, 10.0mmol), potassium carbonate (1.36g, 11.0mmol), bromoisoctane (1.94g, 10.0mmol) in N,N-dimethylformaldehyde Amide (DMF) (30mL), in N 2 Reaction at 150°C for 8h under protection. The mixture was cooled to room temperature, then poured into water (100 mL), extracted with dichloromethane (DCM) (30 mL×3). The organic layers were combined, washed with water (150 mL×3), dried, and the solvent was removed under reduced pressure. The residue was separated by column chromatography using petroleum ether (PE) as the eluent to obtain 2.67 g of a colorless transparent liquid with a yield of 88.0%. 1 H NMR (400MHz, CDCl 3 )δ7.07(d,J=9.7Hz,1H),6.99(s,1H),6.96–6.90(m,1H),3.88(d,J=7.4Hz,2H),1.48–1.25(m,8H ), 0.93 (t, J=7.5Hz, 6H).
[0039] Synthesis of compound M2
[0040] M1 ...
Embodiment 2
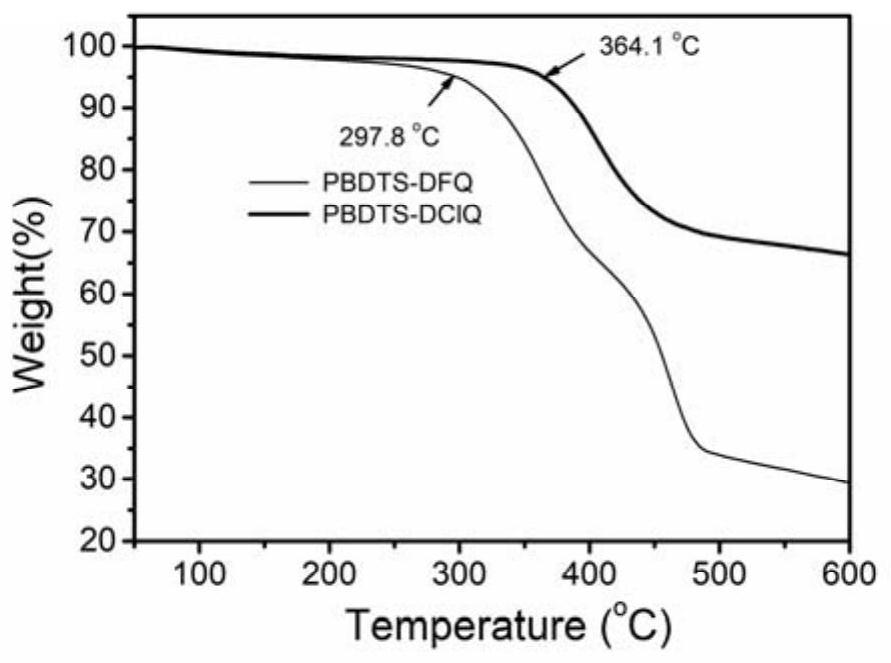
[0066] Thermal stability (TGA) curves of polymers PBDTS-DFQ and PBDTS-DClQ
[0067] figure 1 It is the thermostability (TGA) curve figure of polymer PBDTS-DFQ and PBDTS-DClQ, and the decomposition temperature of PBDTS-DFQ and PBDTS-DClQ is respectively 297.8 and 364.1 ℃ when thermal weight loss 5%, illustrates that two kinds of polymers all have Good thermal stability can meet the needs of device processing.
Embodiment 3
[0069] Crystallinity (DSC) curves of polymers PBDTS-DFQ and PBDTS-DClQ
[0070] The crystallization properties of the polymers PBDTS-DFQ and PBDTS-DClQ were tested by differential scanning calorimetry (DSC), as figure 2 shown. The two polymers have no obvious melting peak and crystallization peak in the temperature range of 40-310 °C, indicating that the two polymers are in an amorphous state.
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com