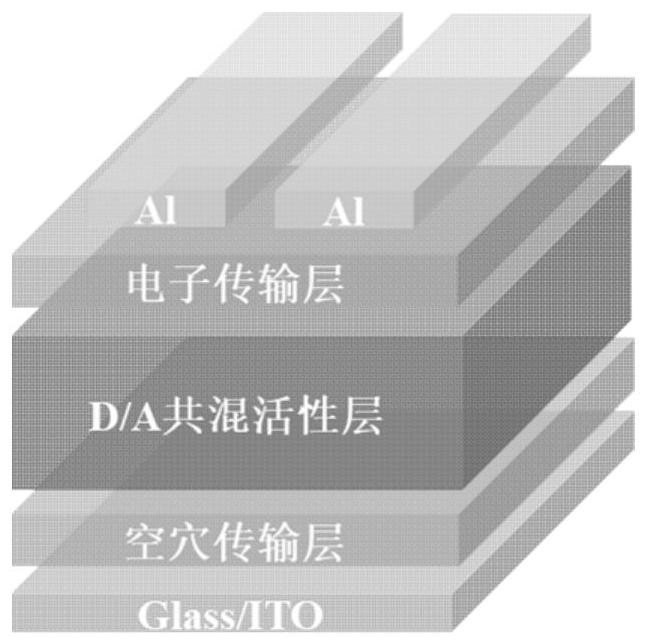
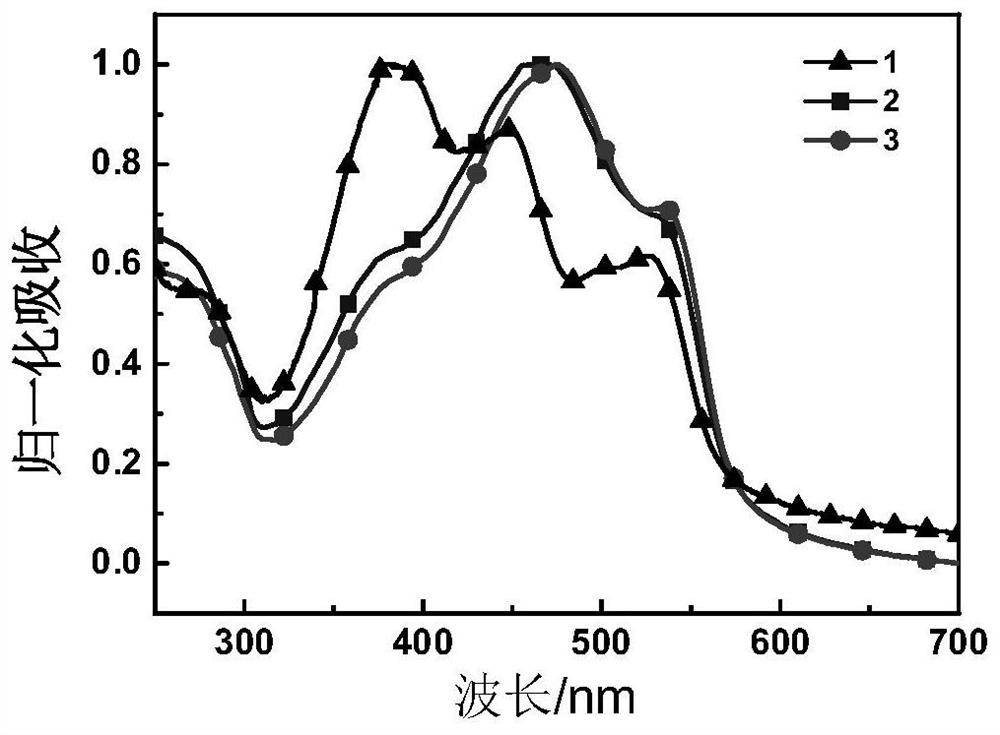
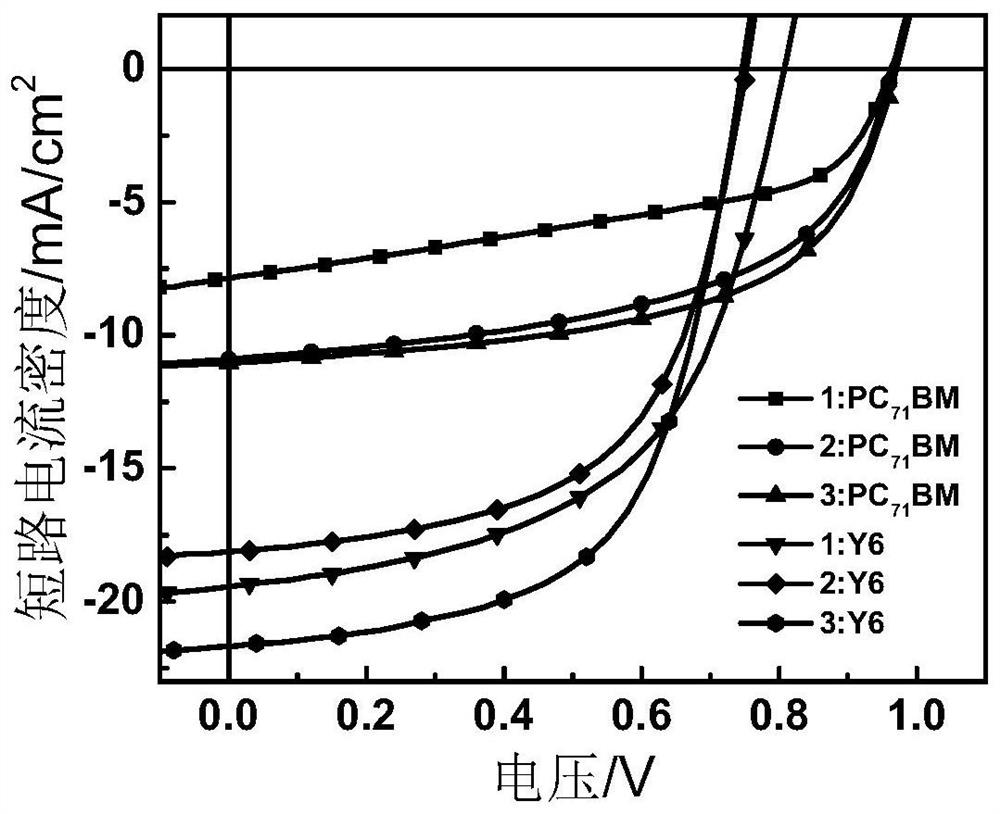
Iridium complex organic photovoltaic material as well as preparation method and application thereof
A technology of organic photovoltaic materials and iridium complexes, applied in organic chemistry, photovoltaic power generation, indium organic compounds, etc., can solve problems such as unsatisfactory device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] In this example, the iridium complex Ir(BzT) with benzothiazole bisthiophene as the main ligand and the terminal side chain as n-hexyl 3 (being called for short compound 1) synthetic, its structural formula is:
[0064]
[0065] Compound (Ir(BzBr) 3 ), 2-(5-hexylthiophen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborane and potassium carbonate in a mass ratio of 1:4:12 Added to a mixed solvent of toluene, ethanol and water with a volume ratio of 5:1:2, the compound (Ir(BzBr) 3 ) at a concentration of 12.5 mg / mL, under a nitrogen atmosphere, tetrakistriphenylphosphine palladium with a total mass of solid reactants of 0.5% was quickly added, and reacted under a nitrogen atmosphere at 100° C. for 24 hours. After the reaction, cool to room temperature and extract with dichloromethane, collect the organic phase, dry over anhydrous sodium sulfate, filter, and spin dry. Using dichloromethane / petroleum ether (v / v)=1:2 as the eluent for column chromatography analysis, the target...
Embodiment 2
[0067] In this example, the iridium complex Ir(tTBz) with benzothiazole-trithiophene as the main ligand and the terminal side chain as tert-butyl 3 (being called for short compound 2) synthetic, its structural formula is:
[0068]
[0069] Compound (Ir(BzBr) 3 ), 2-(5'-(tert-butyl)-[2,2'-bithiophene]-5-yl)-4,4,5,5-tetramethyl-1,3,2-dioxa Borane and potassium carbonate are added to a mixed solvent of toluene, ethanol and water with a volume ratio of 5:1:2 at a mass ratio of 1:4:12, and the compound (Ir(BzBr) 3 ) at a concentration of 12.5 mg / mL, under a nitrogen atmosphere, the catalyst tetrakistriphenylphosphine palladium with a total mass of 0.5% of solid reactants was quickly added, and reacted under a nitrogen atmosphere at 100° C. for 24 hours. After the reaction, cool to room temperature, extract with dichloromethane, collect the organic phase, dry over anhydrous sodium sulfate, filter, and spin dry. Using dichloromethane / petroleum ether (v / v)=1:1 as the eluent for ...
Embodiment 3
[0071] In this example, the iridium complex Ir(TBz) with benzothiazole-trithiophene as the main ligand and the terminal side chain as n-hexyl 3 (being called for short compound 3) synthetic, its structural formula is:
[0072]
[0073] Compound (Ir(BzBr) 3 ), tributyl(5'-hexyl-[2,2'-bithiophene]-5-yl)stannane was added to toluene and N,N-dimethyl with a volume ratio of 10:1 at a mass ratio of 1:4 In a mixed solvent of methyl formamide (DMF), the compound (Ir(BzBr) 3) concentration of 12.5mg / mL, under nitrogen atmosphere, the catalyst tetrakistriphenylphosphine palladium of 2% of the total mass of solid reactants was quickly added, and reacted under nitrogen atmosphere and 110° C. for 12 hours. After the reaction, cool to room temperature, extract with dichloromethane, collect the organic phase, dry over anhydrous sodium sulfate, filter, and spin dry. Using dichloromethane / petroleum ether (v / v)=1:1 as the eluent for column chromatography analysis, the target product compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com