Synthesis and application of an anti-melanoma prodrug activated by a dual bioactive factor cascade
A bioactive factor, anti-melanin technology, applied in the field of organic synthesis, can solve problems such as side effects of melanocytes, and achieve the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
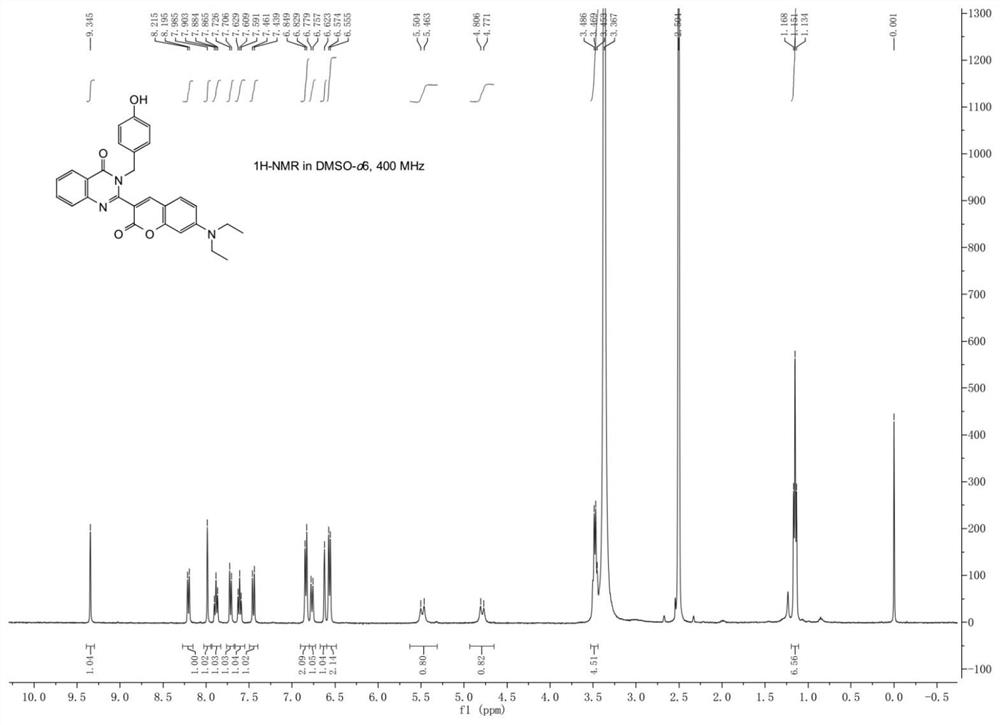
[0041] Compound 1a (4 mmol) and 7-diethylaminocoumarin aldehyde (4 mmol) were dissolved in DMSO (15 mL), and the mixture was reacted at 120°C. After monitoring the completion of the reaction by TLC chromatography, after the reaction mixture was cooled, the system was poured into an appropriate amount of ice water, then the resulting precipitate was filtered off with suction and washed with water three times, recrystallized with ethanol, and the filtered precipitate was vacuum-dried to obtain the compound 2a as an orange-yellow solid (1.31 g, 62.1% yield). 1H NMR (400MHz, Chloroform-d) δ8.37 (dt, J=8.1, 1.1 Hz, 1H), 7.86–7.78 (m, 2H), 7.60–7.54 (m, 2H), 7.33–7.29 (m, 2H) ),7.23(d,J=8.9Hz,1H),7.00–6.94(m,2H),6.63(dd,J=8.9,2.5Hz,1H),6.55(d,J=2.5Hz,1H),5.95 (s, 1H), 5.17(s, 1H), 4.80(s, 1H), 3.49(q, J=7.1Hz, 4H), 1.29(d, J=11.6Hz, 6H).
[0042]
[0043] In a 100mL double neck bottle, in N 2 Under the atmosphere, bis(pinacol)diboron (9 mmol), KOAc (18 mmol) and co...
Embodiment 2
[0045]
[0046] Compound 1a (4 mmol) and 4-pyridinecarbaldehyde (4 mmol) were dissolved in DMSO (15 mL), and the mixture was reacted at 120 °C to obtain compound 2b as a white solid (1.01 g, yield 64.3%). 1 H NMR (400MHz, DMSO-d 6 )δ8.71–8.61(m,2H),8.24(dd,J=8.0,1.5Hz,1H),7.92(ddd,J=8.5,7.2,1.6Hz,1H),7.75(dd,J=8.3, 1.2Hz, 1H), 7.64 (ddd, J=8.1, 7.2, 1.2Hz, 1H), 7.52–7.38 (m, 4H), 6.98–6.87 (m, 2H), 5.12 (s, 2H). 13 C NMR (101MHz, DMSO-d 6 )δ161.63,154.29,150.32,147.26,142.63,136.32,135.40,131.83,129.11,128.16,127.92,126.96,122.90,121.03,120.76,48.04.ESI-MSm / z, calc for C 20 H 14b rN 3 O + [M+H] + :393.25; found, 393.3.
[0047]
[0048] In a 100mL double neck bottle, in N 2 Under the atmosphere, bis(pinacol)diboron (9 mmol), KOAc (18 mmol) and compound 2b (6 mmol) were dissolved in 35 mL of 1,4-dioxane, the system was evacuated 3 times, and then Pd ( dppf)Cl 2 (0.3 mmol) was added to the system, and the vacuum was evacuated twice, and the mixture was refluxed a...
Embodiment 3
[0052]
[0053] In a 100mL single-neck flask, add 15mL THF and isatoic anhydride (12mmol), stir and dissolve at room temperature, then add 3-bromophenethylamino (12mmol) and triethylamine (12mmol), and react the mixture at room temperature for about 3 hours . Column chromatography (CH 2 Cl 2 ) after purification of the crude product gave compound 1c as a yellow solid (1.766 g, 46.1% yield). 1 H NMR (400MHz, DMSO-d 6 )δ8.26(s,1H),7.46(s,1H),7.40(d,J=7.3Hz,2H),7.26(d,J=5.2Hz,2H),7.12(t,J=7.7Hz, 1H), 6.68(d, J=8.2Hz, 1H), 6.49(t, J=7.5Hz, 1H), 6.33(s, 2H), 3.44(q, J=6.8Hz, 2H), 2.84(t, J=7.2Hz, 2H). 13 C NMR (101MHz, DMSO-d 6 )δ169.36,150.01,143.07,132.03,131.93,130.87,129.41,128.41,128.32,122.07,116.76,115.35,115.01,40.56,35.02.ESI-MS m / z,calcd for C 15 H 15b rN 2 O + [M+Na] + :343.2; found, 343.02
[0054]
[0055] Compound 1c (4 mmol) and 7-diethylaminocoumarin aldehyde (4 mmol) were dissolved in DMSO (15 mL), and the mixture was reacted at 120°C to give comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com