A copper complex of polypeptide derivatives, its preparation method and application, and an anticancer drug
A technology of copper complexes and peptide derivatives, applied in the field of biomedicine, can solve problems such as large side effects and drug resistance of tumor cells, and achieve the effects of increasing cellularity, good anti-tumor activity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] In the present invention, the preparation method of (S)-2-decanoylamino-3-(1-naphthyl)propionyl-leucyl-valine preferably comprises the following steps:
[0042] (1) L-Val-OBzl and Boc-Leu undergo the first condensation reaction to obtain Boc-Leu-Val-OBzl;
[0043] (2) The Boc-Leu-Val-OBzl reacts with the first deprotecting reagent to obtain HCl Leu-Val-OBzl;
[0044] (3) Boc-3-(1-naphthyl)-L-alanine carries out the second condensation reaction with the HCl Leu-Val-OBzl to obtain Boc-3-(1-naphthyl)-propionyl- Leucyl-valine benzyl ester;
[0045] (4) The Boc-3-(1-naphthyl)-propionyl-leucyl-valine benzyl ester reacts with the second deprotection reagent to obtain HCl NH 2 -3-(1-naphthyl)-propionyl-leucyl-valine benzyl ester;
[0046] (5) Capric acid and the HCl NH 2 -3-(1-naphthyl)-propionyl-leucyl-valine benzyl ester undergoes a third condensation reaction to obtain (S)-2-decanoylamino-3-(1-naphthyl)propionyl- Leucyl-valine benzyl ester;
[0047] (6) In flow H 2 Un...
Embodiment 1
[0094] Example 1 (S)-2-decanoylamino-3-(1-naphthyl) propionyl-leucyl-valine (abbreviated as C 10 Preparation of NalLeuVal)
[0095] (1) Preparation of Boc-Leu-Val-OBzl
[0096] Weigh 1.15g (5mmol) Boc-Leu, 0.96g (5mmol) EDC, 0.68g (5mmol) HOBt, put them into a stirring bar, add 20mL acetonitrile, activate in ice bath for 20min, weigh 1.35g (5.5mmol) HCl H -Val-OBzl was added to the eggplant bottle, and 2 mL of NMM was added to adjust the pH to 9, and the reaction was carried out at room temperature for 12 hours. The reaction was monitored by TLC (ethyl acetate / petroleum ether 1:2, Rf=0.3), and the raw material point disappeared to terminate the reaction. Remove the organic solvent with a rotary evaporator to obtain a yellow viscous sample, add ethyl acetate, and obtain a light yellow suspension by ultrasonication, add distilled water, and the solution is layered. Pour the mixed solution into a separatory funnel, discard the lower liquid, keep the upper ethyl acetate layer, a...
Embodiment 2
[0116] Example 2 Polypeptide Derivative Copper Complex Cu-C 10 Preparation of NalLeuVal
[0117] ①Weigh 3.4mgCuCl 2 2H 2 O, add 1mL of distilled water to dissolve, the solution is clear and light blue.
[0118] ②Weigh 11.6mg of (S)-2-decanoylamino-3-(1-naphthyl)propionyl-leucyl-valine, add CuCl prepared above 2 In the solution, ultrasonic 30min, the solution is milky.
[0119]③ After the above emulsion was freeze-dried at -20°C for 10 hours with a freeze dryer, a yellow-green solid was obtained, which was the polypeptide derivative copper complex Cu-C 10 NalLeuVal, denoted as C10NLVCu.
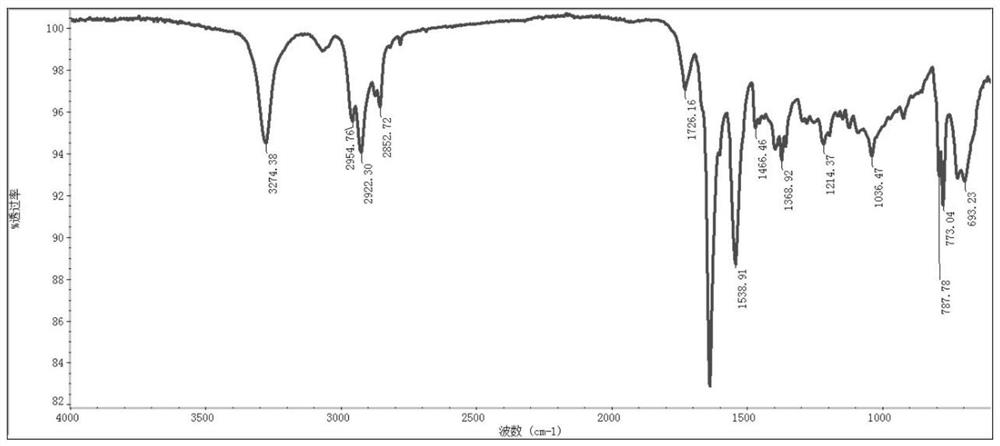
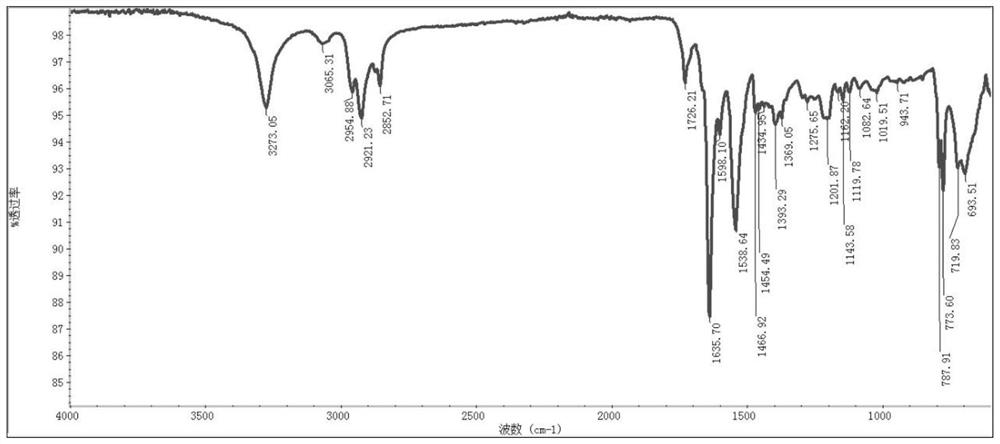
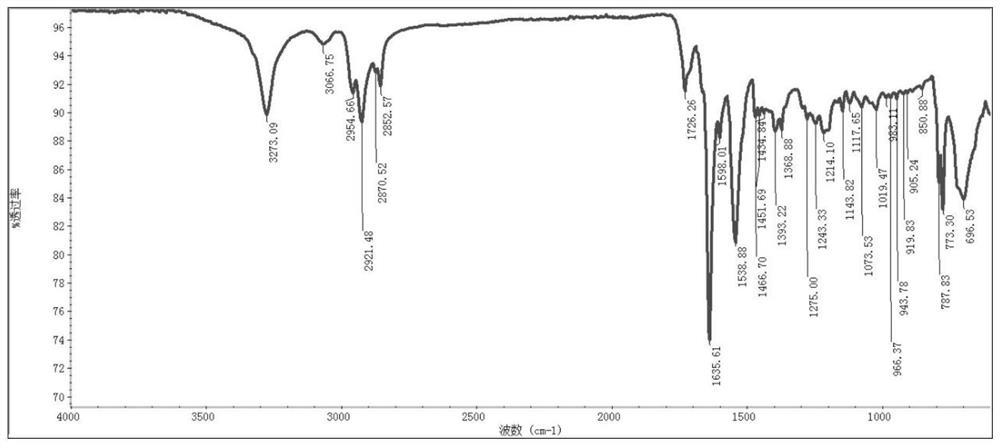
[0120] The obtained polypeptide derivative copper complex Cu-C 10 The infrared spectrum of NalLeuVal is as follows figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com