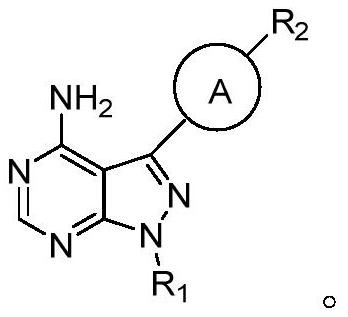
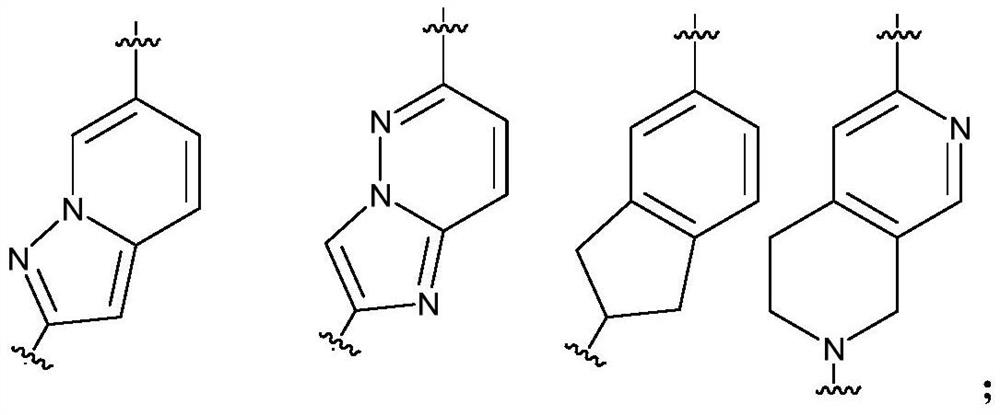
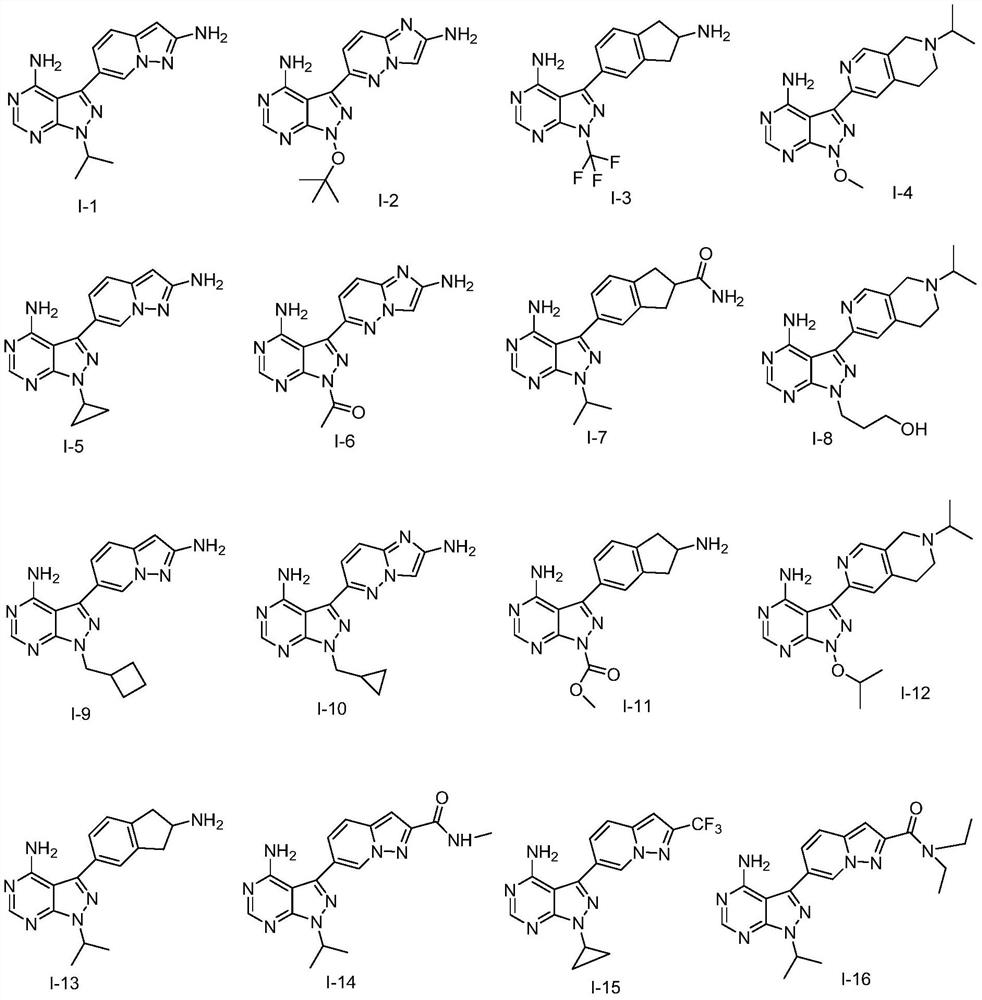
Pyrazolopyrimidines as Kinase Inhibitors and Applications
A technology for pyrazolopyrimidines and compounds, which is applied in the field of pyrazolopyrimidine compounds and can solve the problems of easy generation of drug resistance, many adverse reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 compound I-1
[0059]
[0060] Under the protection of nitrogen, the compound shown in formula I-A1 (21.2g, 0.1mol), the compound shown in formula I-B1 (77.9g, 0.44mol), tetrakis triphenylphosphine palladium (4.6g, 0.004mol), Potassium carbonate (121g, 0.88mol), 800 milliliters of toluene, 600 milliliters of ethanol, add in the reactor and stir evenly, slowly heat up to 60~70 ℃ of reaction, through thin-layer chromatography (TLC) spot plate test reaction is complete, then drop to At 20°C, add 1000 ml of water and 1000 ml of dichloromethane, stir, separate layers, wash the toluene layer twice with water, dry over anhydrous sodium sulfate, filter to remove the desiccant, then pass through a silica gel column, and concentrate the eluent to dryness under reduced pressure. That is, 21.4 g of solid powder (69.4% yield) was used to obtain the product compound I-1 with a purity of 99.0% (HPLC).
[0061] LC / MS: [M+H]+309.
Embodiment 2
[0062] The preparation of embodiment 2 compound I-23
[0063]
[0064] Under nitrogen protection, the compound shown in formula I-A1 (21.2g, 0.1mol), the compound shown in formula I-B23 (95.5g, 0.44mol), tetrakis triphenylphosphine palladium (4.6g, 0.004mol), Potassium carbonate (121g, 0.88mol), 800 milliliters of toluene, 600 milliliters of ethanol, add in the reactor and stir evenly, slowly heat up to 60~70 ℃ of reaction, through thin-layer chromatography (TLC) spot plate test reaction is complete, then drop to At 20°C, add 1000 ml of water and 1000 ml of dichloromethane, stir, separate layers, wash the toluene layer twice with water, dry over anhydrous sodium sulfate, filter to remove the desiccant, then pass through a silica gel column, and concentrate the eluent to dryness under reduced pressure. That is, 25.1 g of solid powder (yield 72.0%) was used to obtain the product compound I-23 with a purity of 99.2% (high performance liquid phase).
[0065] LC / MS: [M+H]+349. ...
Embodiment 3
[0066] The preparation of embodiment 3 compound I-25
[0067]
[0068] Under the protection of nitrogen, the compound shown in formula I-A25 (24.2g, 0.1mol), the compound shown in formula I-B25 (96.8g, 0.44mol), tetrakistriphenylphosphine palladium (4.6g, 0.004mol), Potassium carbonate (121g, 0.88mol), 800 milliliters of toluene, 600 milliliters of ethanol, add in the reactor and stir evenly, slowly heat up to 60~70 ℃ of reaction, through thin-layer chromatography (TLC) spot plate test reaction is complete, then drop to At 20°C, add 1000 ml of water and 1000 ml of dichloromethane, stir, separate layers, wash the toluene layer twice with water, dry over anhydrous sodium sulfate, filter to remove the desiccant, then pass through a silica gel column, and concentrate the eluent to dryness under reduced pressure. That is, 28.6 grams of solid powder (yield 74.9%) obtained the product compound I-25 with a purity of 99.5% (high performance liquid phase).
[0069] LC / MS: [M+H]+382....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com