Method for synthesizing quinoxaline compound under visible light induced iron catalysis condition
A visible light and quinoxaline technology, applied in organic chemistry and other fields, can solve difficult problems and achieve excellent efficiency and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 2-methylquinoxaline
[0037] Get a quartz reaction tube, add a magnetic stirrer therein, then add 0.3mmol o-phenylenediamine, 0.6mmol tri-n-propylamine, 10mmol% photosensitizer iron salt (Fe 3 o 4 ), and finally 1 mL of dimethyl sulfoxide (DMSO) was added.
[0038] Then add a three-way gas guide head with a balloon above the quartz reaction tube, first use liquid nitrogen to freeze the reaction stock solution completely, then use an oil pump to evacuate the quartz reaction tube, and then fill the balloon with oxygen; stir with a magnetic stirrer Under the condition of oxygen, the reaction was carried out under the irradiation of xenon lamp (400-780nm) light for 48h, the final product was detected by TLC, and finally the final product 2-methylquinoxaline was obtained by separation by column chromatography with a yield of 73%. The reaction equation is as follows:
[0039]
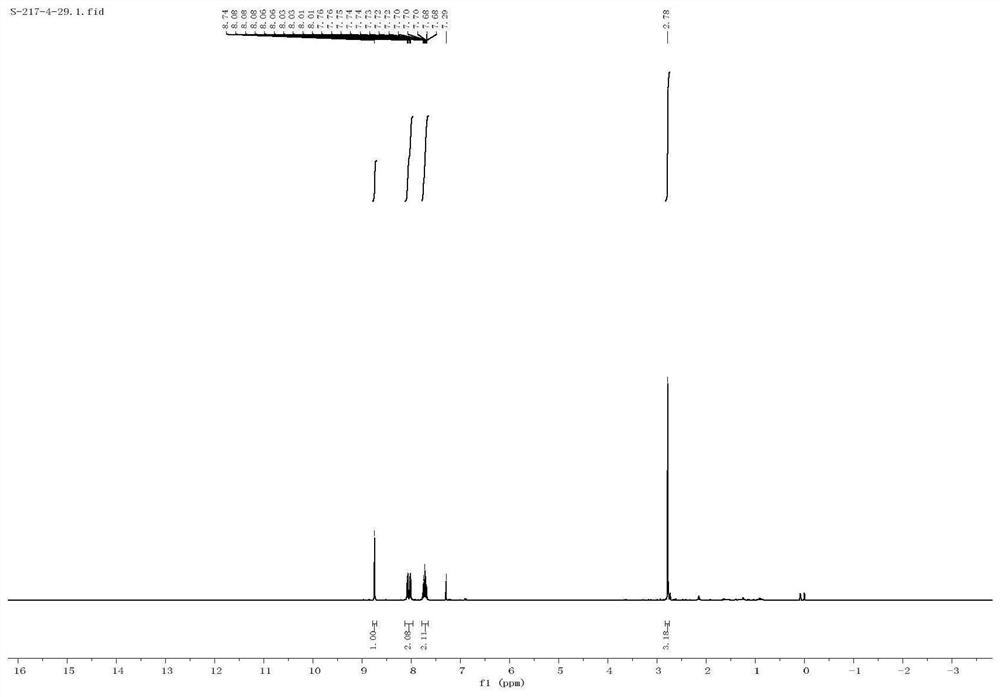
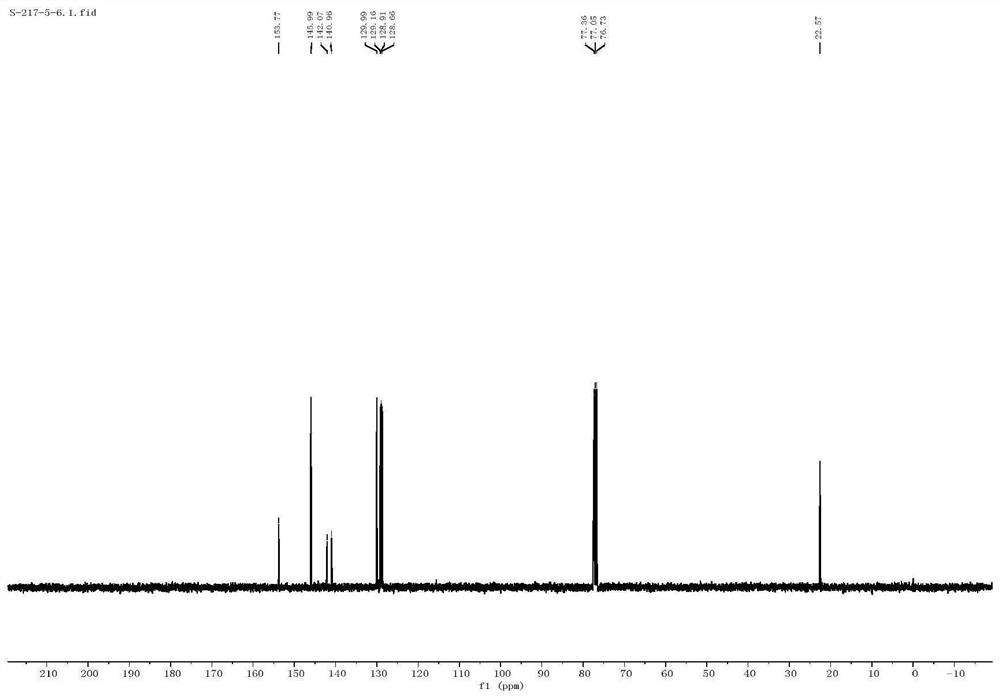
[0040] The proton nuclear magnetic resonance spectrum characterization of 2-me...
Embodiment 2
[0043] Preparation of 2-Propylquinoxaline
[0044] Get a quartz reaction tube, add a magnetic stirrer therein, then add 0.3mmol o-phenylenediamine, 0.6mmol tripentylamine, 10mmol% photosensitizer iron salt (Fe(NO 3 ) 3 ), and finally 1 mL of dimethyl sulfoxide (DMSO) was added.
[0045] Then add a three-way gas guide head with a balloon above the quartz reaction tube, first use liquid nitrogen to freeze the reaction stock solution completely, then use an oil pump to evacuate the quartz reaction tube, and then fill the balloon with oxygen; stir with a magnetic stirrer Under oxygen conditions, the reaction was performed under blue LED light for 48 hours, the final product was detected by TLC, and finally the final product 2-propylquinoxaline was obtained by separation by column chromatography with a yield of 70%. The reaction equation is as follows:
[0046]
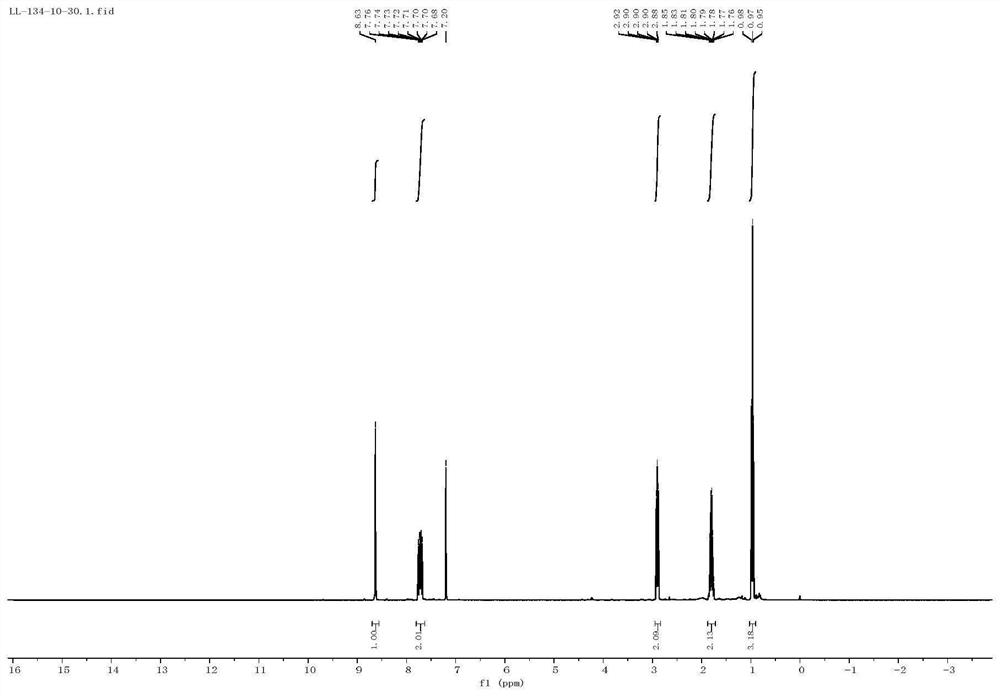
[0047] 2-Propylquinoxaline H NMR spectrum is characterized as follows: 8.65 (s, 1H), 8.05-7.84 (m, 2H), 7.70-7.54 ...
Embodiment 3
[0049] Preparation of 2-butyl-6,7-dihydroquinoxaline
[0050] Get a quartz reaction tube, add a magnetic stirrer thereto, then add 0.3mmol o-phenylenediamine, 0.6mmol trihexylamine, 10mmol% photosensitizer iron salt (FeSO 4 ), and finally 1 mL of dimethyl sulfoxide (DMSO) was added.
[0051] Then add a three-way gas guide head with a balloon above the quartz reaction tube, first use liquid nitrogen to freeze the reaction stock solution completely, then use an oil pump to evacuate the quartz reaction tube, and then fill the balloon with oxygen; stir with a magnetic stirrer Under the condition of oxygen, it was reacted for 48h under the irradiation of xenon lamp (400-780nm) light, the final product was detected by TLC, and finally the final product 2-butyl-6,7-dihydroquinoxaline was obtained by column chromatography separation, producing The rate is 77%. The reaction equation is as follows:
[0052]
[0053] The proton nuclear magnetic resonance spectrum of 2-butyl-6,7-dih...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com