Nitrogen-containing compound and electronic component and electronic device containing it
A nitrogen compound and chemical bond technology is applied in the fields of nitrogen-containing compounds and electronic components and electronic devices containing them to achieve the effects of improving electron transport performance, increasing polarity and high bond energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
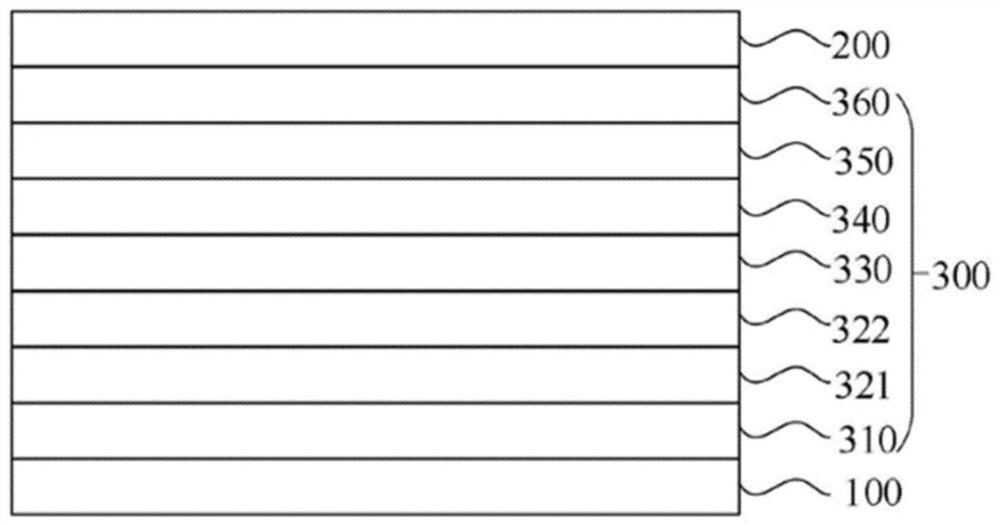
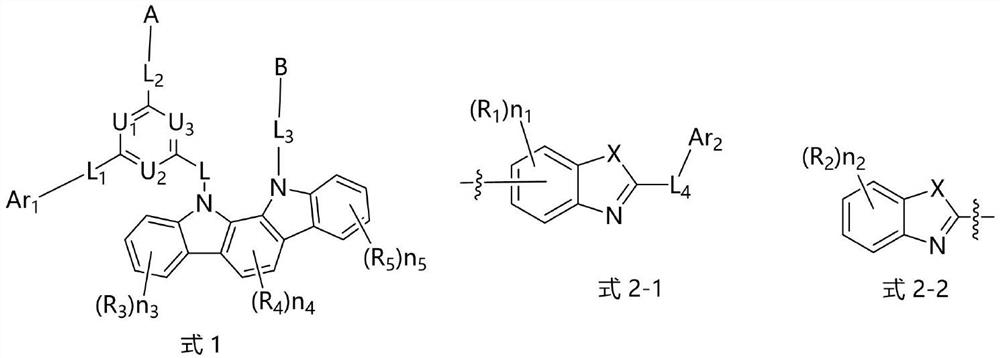
Image
Examples
preparation example 1
[0167] Preparation Example 1: Synthesis of Compound 67
[0168] (1) Synthesis of reactant B-1
[0169]
[0170] Nitrogen gas (0.100L / min) was introduced into the three-necked flask equipped with mechanical stirring, thermometer and spherical condenser for 15 minutes, and 2-bromo-6-nitrophenol (50.0g, 229.3mmol), benzyl alcohol (29.76 g, 275.2mmol), 1,1'-bis(diphenylphosphino)ferrocene (3.71g, 6.8mmol) and xylene (500mL) started stirring and heating, when the temperature rose to 125-135°C, reflux reaction 36h, stop stirring and heating after the reaction is completed, and start to process the reaction when the temperature drops to room temperature; add toluene and water to extract the reaction solution, combine the organic phase, dry the organic layer with anhydrous magnesium sulfate, filter, and concentrate; use dichloromethane The crude product was purified by silica gel column chromatography / n-heptane system to obtain solid compound reactant B-1 (40.23 g, 64%).
[0171]...
preparation example 28
[0192] Preparation 28: Synthesis of Compound 257
[0193] (1) Synthesis of intermediate Sub 1-II-A11
[0194]
[0195] Nitrogen (0.100L / min) was introduced into a three-neck flask equipped with mechanical stirring, a thermometer, and a spherical condenser for replacement for 15 minutes, and 2,5-dichlorobenzoxazole (35.0 g, 186.1 mmol) was added (reactant B- 15), 2-naphthylboronic acid (32.0, 186.1mmol) (reactant A-8), potassium carbonate (64.3g, 465.4mmol), tetrakis (triphenylphosphine) palladium (4.3g, 3.7mmol), tetrabutyl Ammonium bromide (1.2 g, 3.72 mmol), and a mixed solvent of toluene (280 mL), ethanol (70 mL) and water (70 mL) was added. Start stirring and heating, wait until the temperature rises to 75-80°C, reflux for 15 hours, and cool to room temperature after the reaction is completed. Extract and separate the organic phase with toluene and water, wash with water until neutral, dry the organic phase with anhydrous magnesium sulfate, filter and concentrate the ...
preparation example 38
[0205] Preparation 38: Synthesis of Compound 121
[0206] (1) Synthesis of intermediate Sub A-19
[0207]
[0208] Nitrogen (0.100L / min) was passed into a three-necked flask equipped with mechanical stirring, a thermometer, and a spherical condenser for replacement for 15 minutes, and indo[2,3-A]carbazole (50.0 g, 195.1 mmol), bromobenzene ( 27.5g, 175.5mmol) (reactant D-1), Pd 2 (dba) 3 (3.5g, 3.9mmol), tri-tert-butylphosphine (1.6g, 7.8mmol), sodium tert-butoxide (41.2g, 429.2mmol), xylene (500mL). Start stirring and heating, wait until the temperature rises to 135-145 ° C, reflux reaction for 10 h, after the reaction is completed, cool to room temperature. Add toluene and water to extract the reaction solution, use anhydrous magnesium sulfate to dry the organic phase, filter and concentrate the filtrate by distillation under reduced pressure, and use dichloromethane / n-heptane system to carry out silica gel column chromatography on the crude product to obtain the solid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com