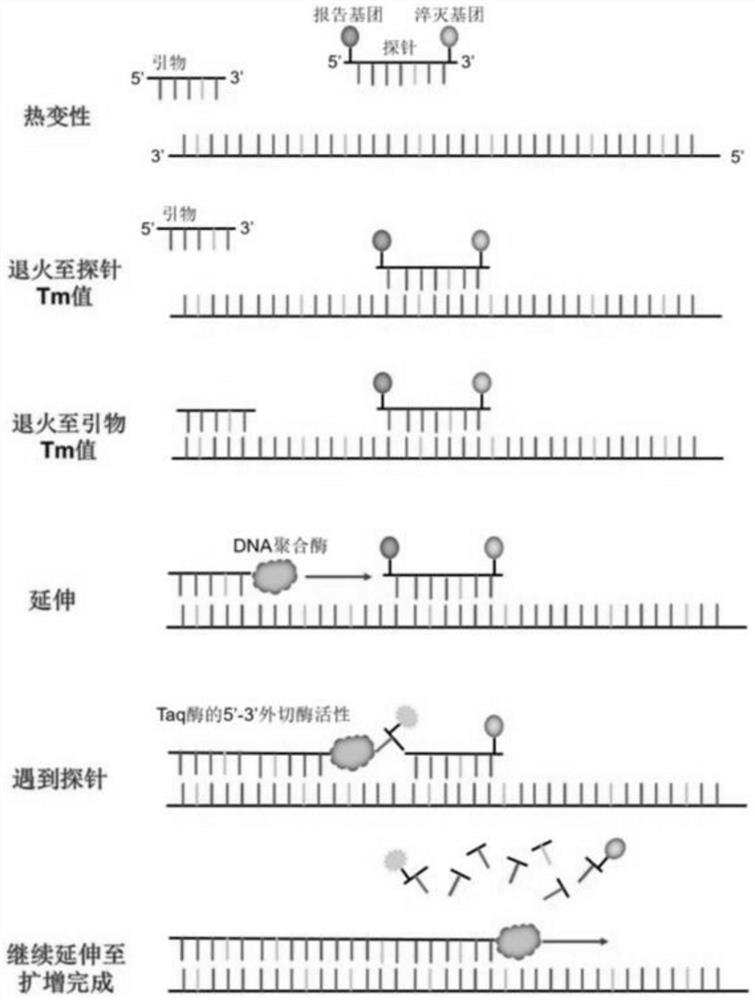
Selenium or thiothymine nucleoside-5 '-triphosphoric acid and synthesis method thereof
A technology of thiothymidine and thymidine, which is applied in the fields of modified triphosphate synthesis and nucleic acid molecule detection, can solve the problems of accurate detection by instruments, flooding, weak signal of low-copy nucleic acid molecules, etc., and achieves pairing accuracy The effect of improving, improving accuracy and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
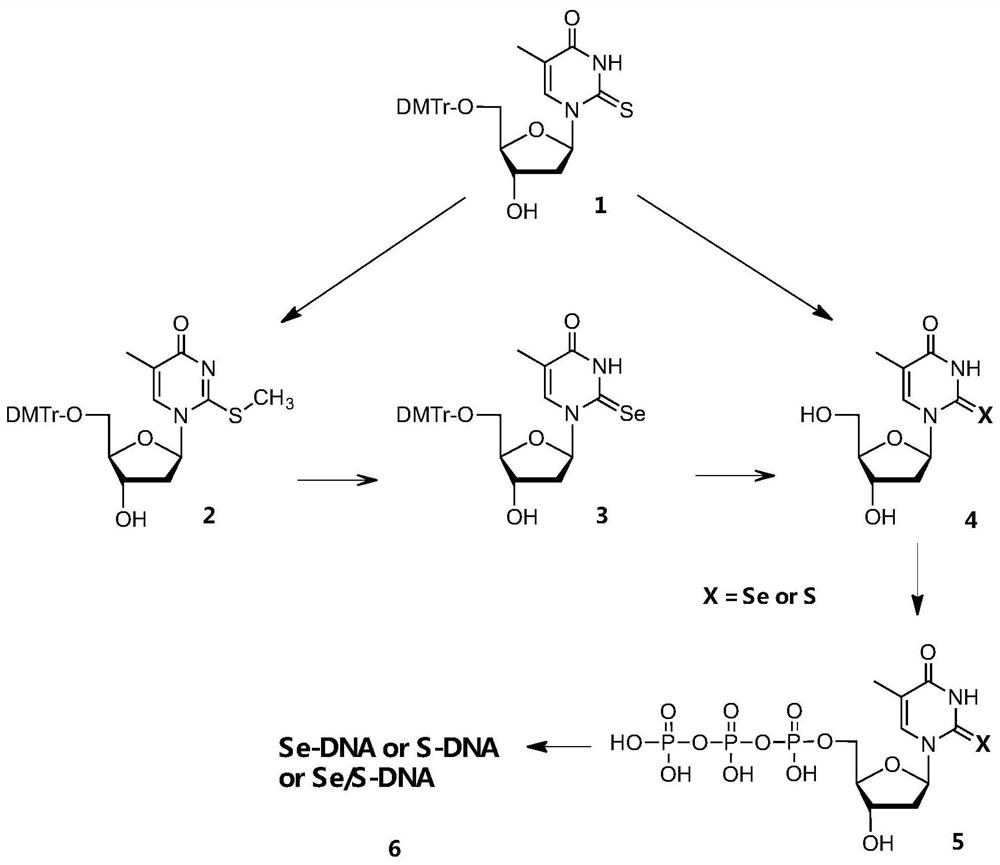
[0068] according to Figure 1-13 As shown, this embodiment provides a kind of selenothymidine-5'-triphosphate and its synthesis method, which is characterized in that it comprises the following synthesis steps:
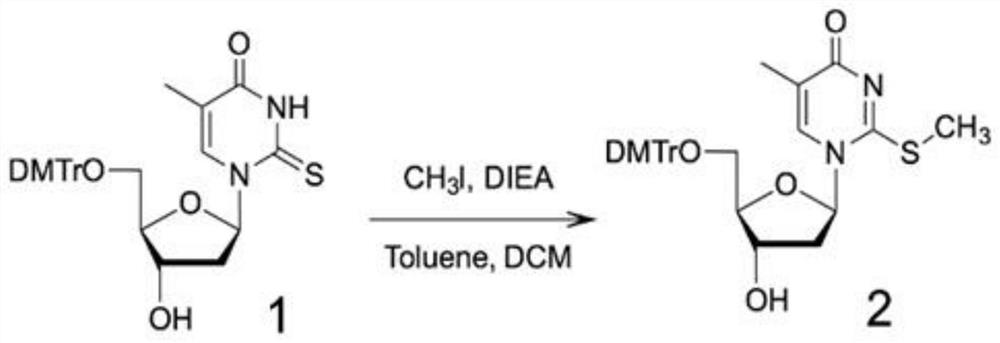
[0069] Step 1: Using 5′-DMTr-2-thiothymidine as compound 1, using CH 3Alkylation of the 2-thiol functional group by I to activate the 2-thiol moiety affords compound 2
[0070] Step 2: Compound 2 was reacted with freshly prepared NaSeH to complete the selenium substitution, and compound 3 was obtained in 80% yield
[0071] Step 3: Compound 3 is deprotected with trichloroacetic acid to obtain Se T, the compound 4
[0072] Step 4: Conversion of compound 4Se to Se TTP, the compound 5Se
[0073] Step 5: Purify compound 5Se and characterize it by nuclear magnetic resonance, mass spectrometry and high performance liquid chromatography to confirm its structure and purity, and then use Se TTP and DNA polymerase for DNA enzymatic synthesis, the DNA polymerase can use Kle...
Embodiment 2
[0099] Such as figure 1 As shown, this example provides a chemical synthesis method of thiothymidine-5'-triphosphate, comprising the following synthesis steps:
[0100] Step 1: using 5'-DMTr-2-thiothymidine as the initial reactant, that is, compound 1, deprotecting reaction between compound 1 and trichloroacetic acid to obtain S T, that is, compound 4S;
[0101] Step 2: Transform compound 4S into S TTP, namely compound 5S;
[0102] Step 3: Characterize compound 5S after purification to confirm its structure and purity, and then use S TTP and DNA polymerase carry out DNA enzymatic synthesis; The present invention is through the method of innovative synthesis triphosphate, for example 2-thiothymidine-5'-triphosphate ( S TTP) established a sulfur atom-specific modification strategy (SAM, Sulfur Atom-specific Modification of Nucleic Acids) to suppress T / G mismatches in DNA polymerization, improve the specificity of base pairing, and proved that the SAM method can improve polym...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com