Lignan compound derived from radix paeoniae rubra and preparation method and application thereof
A compound and composition technology, applied in the field of medicine, can solve problems such as allergic diseases of Radix Paeoniae Rubra extract, and patent literature of preparation method of Radix Paeoniae Rubra compound monomer.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
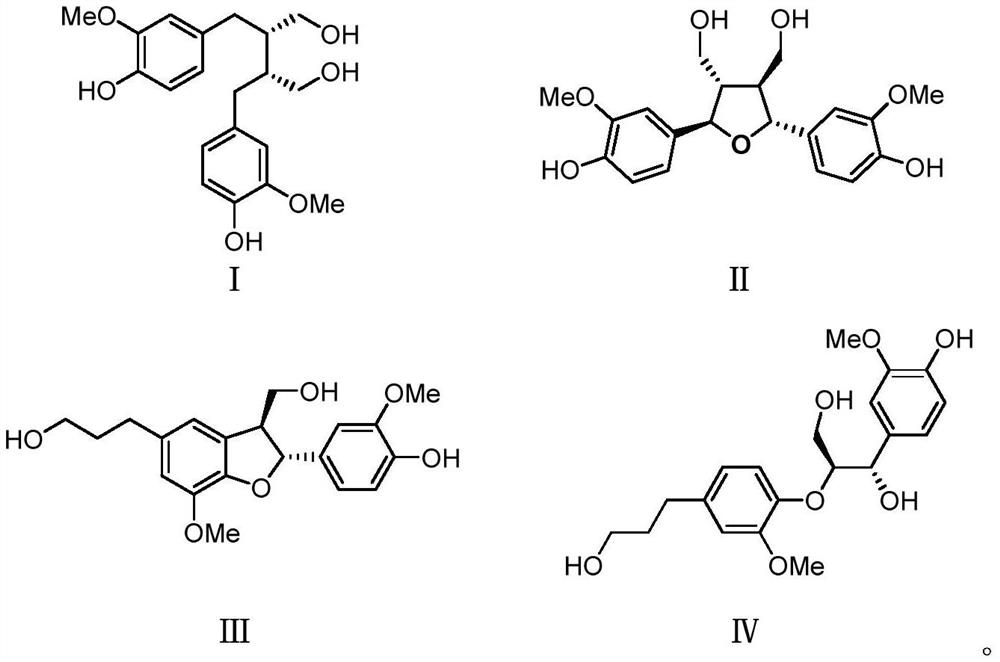
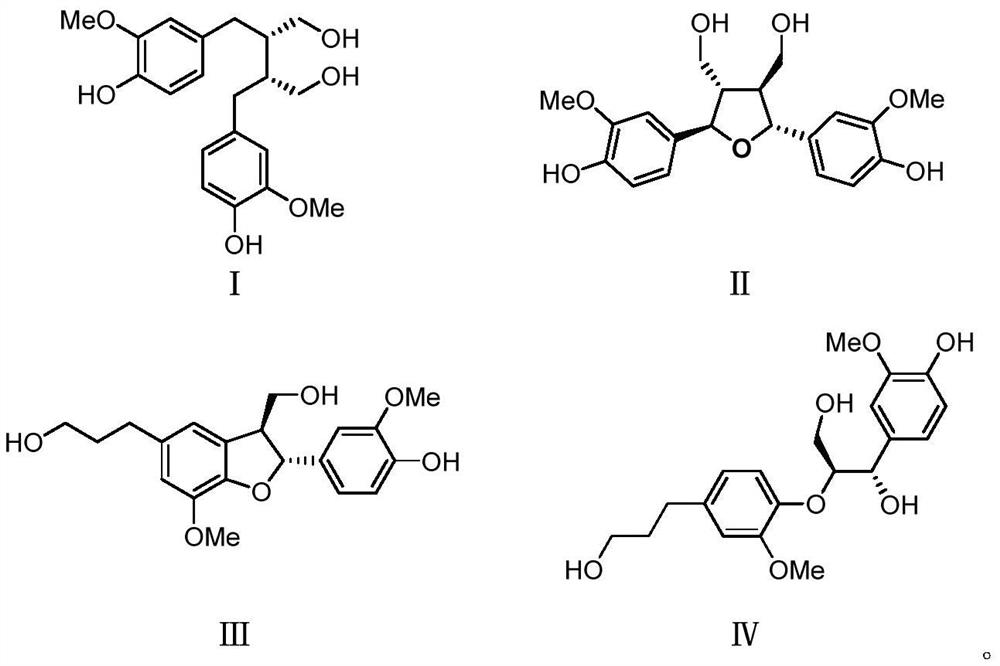
[0037] Embodiment 1, the preparation of compound I, II, III, IV
[0038] Radix Paeoniae Rubra decoction pieces 50kg, soaked in distilled water and ultrasonically extracted 3 times, 1 hour each time. The water extract was directly separated by macroporous adsorption resin, eluted with 50% ethanol, and the eluate was concentrated under reduced pressure. The part eluted with 50% ethanol continues to be separated by MCI column, eluted with 30% ethanol and 50% ethanol in turn, and the eluate is concentrated under reduced pressure, and the sample eluted with 30% ethanol is CS-3 (2000g), 50% ethanol The eluted sample was CS-4 (316 g). The residue was concentrated to no alcohol by 95% ethanol extract, extracted 5 times with an equal volume of ethyl acetate, and the extract was concentrated under reduced pressure to obtain the ethyl acetate part CS-1 (580 g) and the water phase part CS-2 (270 g). Such as figure 1 .
[0039] Our previous activity screening found that only CS-4 has a...
experiment example 1
[0051] Exploration of Cytotoxicity of Parts of Radix Paeoniae Rubra
[0052] The RBL-2H3 cells in the logarithmic growth phase were taken out from the incubator, and the cells were digested to prepare a cell suspension. Cell density was calculated using a handheld cell counter (model: scepter). Adjust the cell number to 1×10 with fresh complete medium 5 cells / ml, inoculate in 96-well plate, 200 μL per well, mix the cell suspension once for every three wells inoculated, after culturing for 24 hours, discard the supernatant, and add different concentrations (0.2 μg / ml , T2 is 2 μg / ml, T3 is 20 μg / ml) drug to be screened, 3 duplicate wells in each group, and a normal group (cell blank wells without drug addition) and zero adjustment wells (blank wells without inoculating cells), After culturing for 24 hours, discard the supernatant, add 200 μL of serum-free MTT solution (serum-free medium: 5 mg / ml: MTT=1:10), incubate for 4 hours, centrifuge at 400 g / 5 min, discard the supernat...
experiment example 2
[0058] Study on the effect of Radix Paeoniae Rubra on the degranulation of RBL-2H3 cells caused by allergic reaction
[0059] Digest the cells in the logarithmic growth phase and adjust the cell density to 1×10 5 pieces / ml. 200 μL / well was added into the 96-well plate, and the zero adjustment well, the blank control well, the total enzyme well and each administration well were set. The administration wells were divided into model control group, T1, T2 and T3 groups, wherein the final administration concentration of T1 was 0.2 μg / ml, T2 was 2 μg / ml, and T3 was 20 μg / ml. Incubate overnight, add complete medium to the zero wells, normal wells and total enzyme wells for normal culture, add 200 μL of anti-DNP-IgE prepared in complete medium with a final concentration of 750ng / ml in the model group, and add drugs of various concentrations to the administration wells and anti-DNP-IgE with a final concentration of 750ng / ml in a total of 200 μL, incubated for 24 hours, centrifuged, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com