Oral film compositions and dosage forms having precise active dissolution profiles
An active substance and oral film technology, which is applied in the field of oral film compositions and dosage forms with precise active substance dissolution profiles, and can solve problems such as inability to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0285] Embodiment 1-clobazam
[0286] Oral films were prepared with the following compositions shown in Table 1 to compare ground and micronized actives.
[0287] Table 1 : Formulations of Oral Films Containing Milled or Micronized Clobazam
[0288]
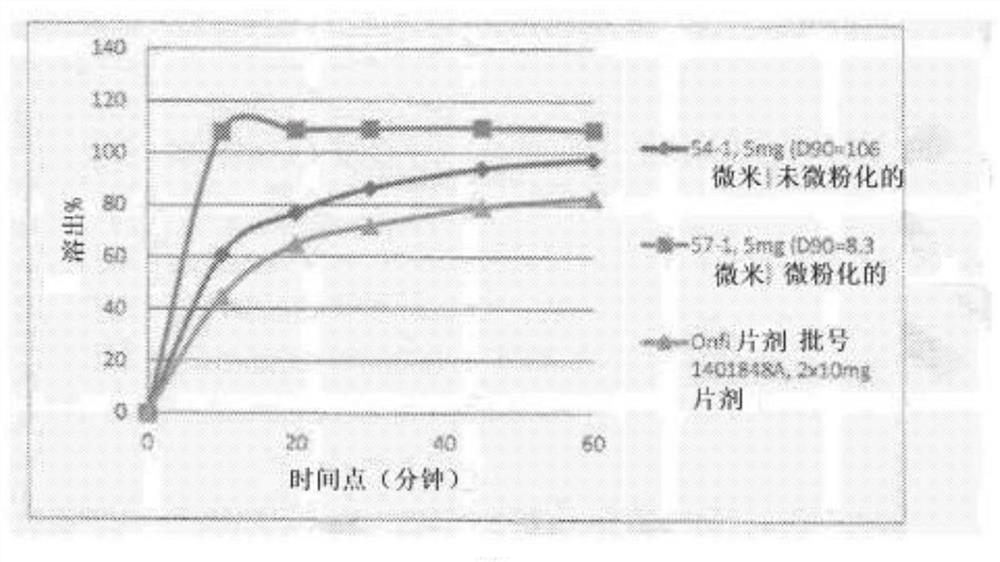
[0289] image 3Mean active dissolution profiles of films (N=3) containing milled (D90=106 microns) and micronized (D90=8.3 microns) clobazam and RLD Onfi tablets are shown. Table 2 shows image 3 Some of the graphically depicted data points shown in .
[0290] Table 2: Dissolution Testing for Oral Films Containing Milled or Micronized Clobazam
[0291]
[0292] Thus, oral films comprising micronized clobazam performed better (ie, dissolved significantly faster) than oral films comprising milled clobazam.
Embodiment 2
[0293] Example 2 - Dissolution Study of Oral Film Containing Diazepam
[0294] Diazepam-containing oral films (DBSF) were prepared containing 5 mg and 15 mg of active. The dissolution of 5 mg and 15 mg of DBSF under storage conditions of 12 months at 25°C was tested using conventional dissolution methods and PION technology. The traditional dissolution method used herein is a common method for testing the dissolution characteristics of solid oral dosage forms such as tablets and capsules. The traditional dissolution method utilizes a modified USP apparatus 5 setup, with modified sample holders necessary to keep the membrane immobilized. All other aspects of traditional dissolution methods are similar to those of other solid oral dosage forms, including manual sampling and collection of samples at specific time points, followed by off-line analysis of the samples using HPLC. Traditional dissolution methods are practically limited by the frequency and consistency of manual sam...
Embodiment 3
[0304] Example 3 - Dissolution Study of Oral Films Containing Riluzole
[0305] An oral film (ROSF) containing 50 mg of riluzole was prepared. The dissolution of 50 mg of ROSF after storage at 25°C for about 6 months was tested in 0.1 N HCl (a medium that simulates gastric conditions) using conventional dissolution methods and PION technology. Table 3 above shows the parameters used for the conventional dissolution method and the PION technique for testing ROSF.
[0306] Image 6 is a graph of the active dissolution profile of ROSF as measured by conventional dissolution testing. Figure 7 is a graph of the active substance dissolution profile of ROSF measured by the PION technique. Table 6 presents a number of data points and a comparison of the results obtained by traditional dissolution methods with those obtained using the PION technology.
[0307] Table 6: Active Dissolution Rates and Comparisons for ROSF
[0308]
[0309] According to the calculations presented i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com