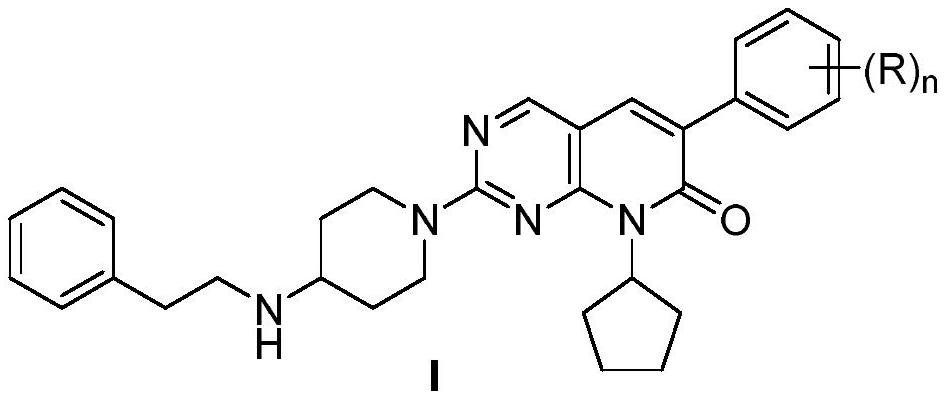
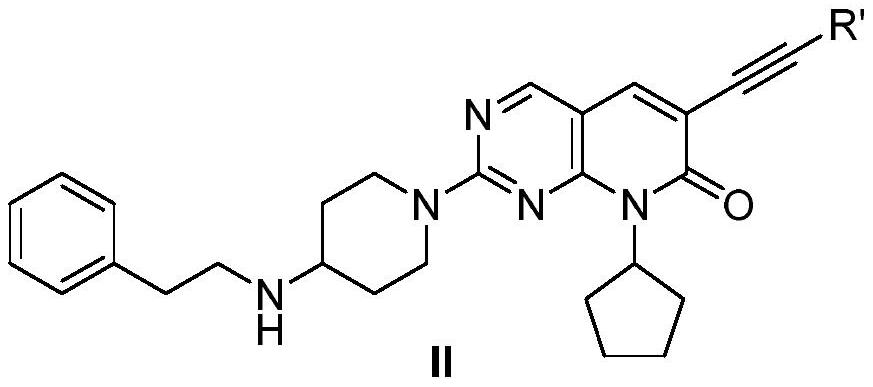
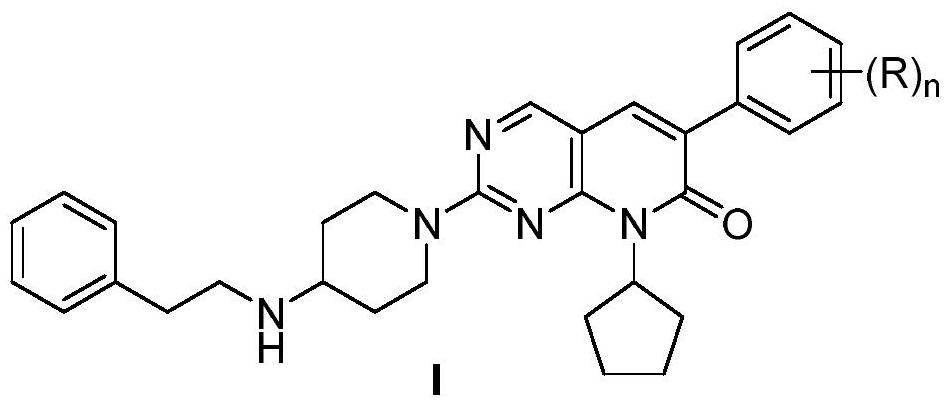
Coumarin compounds as well as preparation method and application thereof
A technology of coumarins and compounds, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: 8-cyclopentyl-2-(4-(phenethylamino)piperidin-1-yl)-6-(3,4-dimethylphenyl)pyrido[2,3-d ] Synthesis of pyrimidin-7-one (compound 1).
[0053]
[0054] synthetic route:
[0055]
[0056] Step 1 Synthesis of compound 1-1: Add the raw material 4-chloro-2-methylthiopyrimidine-5-carboxylate ethyl ester (36.5g, 156.9mmol) into a 500mL round bottom flask, dissolve it in 300mLTHF, and then add cyclopentyl Amine (20.0 g, 235.3 mmol) and triethylamine (31.8 g, 313.8 mmol) were stirred at room temperature for 1 h, and the reaction was complete. Filtrate, remove THF by distilling off the filtrate under reduced pressure, add 300mL ethyl acetate to dissolve, and use saturated NH 4 Cl solution was washed (200mL*3), then washed with saturated brine (200mL*3), and the organic phase was washed with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was evaporated under reduced pressure to remove the solvent, and then 20 mL of toluene was added and concentrate...
Embodiment 2
[0075] Example 2: 8-cyclopentyl-2-(4-(phenethylamino)piperidin-1-yl)-6-phenylpyrido[2,3-d]pyrimidin-7-one (compound 2 ) Synthesis.
[0076]
[0077] synthetic route:
[0078]
[0079] Referring to the preparation method of Example 1, the difference is that compound 1-11 is reacted with phenylboronic acid to obtain compound 2-1, and then compound 2-1 is reacted with trifluoroacetic acid to obtain compound 2.
[0080] 1 H NMR (500MHz, CDCl 3 )δ: 9.73(s, 1H), 8.42(s, 1H), 7.60(d, J=7.5Hz, 2H), 7.49(s, 1H), 7.39(t, J=7.5Hz, 2H), 7.22- 7.34(m, 4H), 7.17(d, J=7Hz, 2H), 5.89(t, J=8.8Hz, 1H), 4.98(d, J=13.5Hz, 2H), 3.30(s, 1H), 3.20 (s,2H),2.94-3.05(m,4H),2.37(s,2H),2.20(d,J=5Hz,2H),2.01(s,2H),1.78(q,J=13.7Hz,4H ), 1.64(d,J=4Hz,2H).
[0081] HRMS(ESI)(m / z):494.2911[M+H] + , calculated for C 31 h 35 N 5 O,493.28382.
Embodiment 3
[0082] Example 3: 8-cyclopentyl-6-(4-fluoro-3-methylphenyl)-2-(4-(phenethylamino)piperidin-1-yl)pyrido[2,3- d] Synthesis of pyrimidin-7-one (compound 3).
[0083]
[0084] synthetic route:
[0085]
[0086] Referring to the preparation method of Example 1, the difference is that compound 3-1 is obtained by reacting compound 1-11 with 4-fluoro-3-methylphenylboronic acid. Compound 3-1 is then reacted with trifluoroacetic acid to obtain compound 3.
[0087] 1 H NMR (500MHz, CDCl 3 )δ:9.73(s,1H),8.42(s,1H),7.44(t,J=6.5Hz,2H),7.37(d,J=5Hz,1H), 7.22-7.31(m,3H),7.17 (d, J=7Hz, 2H), 7.02(t, J=9Hz, 1H), 5.88(t, J=9Hz, 1H), 4.98(d, J=13.5Hz, 2H), 3.30(s, 1H) ,3.20(s,2H),3.02(q,J=8.7Hz,2H),2.96(d,J=12.5Hz,2H),2.36(q,J=6.2Hz,2H), 2.31(d,J= 10Hz, 3H), 2.21(d, J=11.5Hz, 2H), 2.01(s, 2H), 1.76-1.84(m, 4H), 1.64(d, J=5Hz, 2H).
[0088] HRMS(ESI)(m / z):526.2981[M+H] + , calculated for C 32 h 36 FN 5 O,525.29083.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com