Preparation method of lithium bis (fluorosulfonyl) imide
A technology of lithium bisfluorosulfonyl imide and bisfluorosulfonyl imide, which is applied in the field of preparation of lithium bisfluorosulfonyl imide, can solve the problems of inability to obtain high-quality products, difficulty in separation of final products, and difficult separation of intermediates and other issues, to achieve the effect of improving the catalytic effect, high quality, and sufficient contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
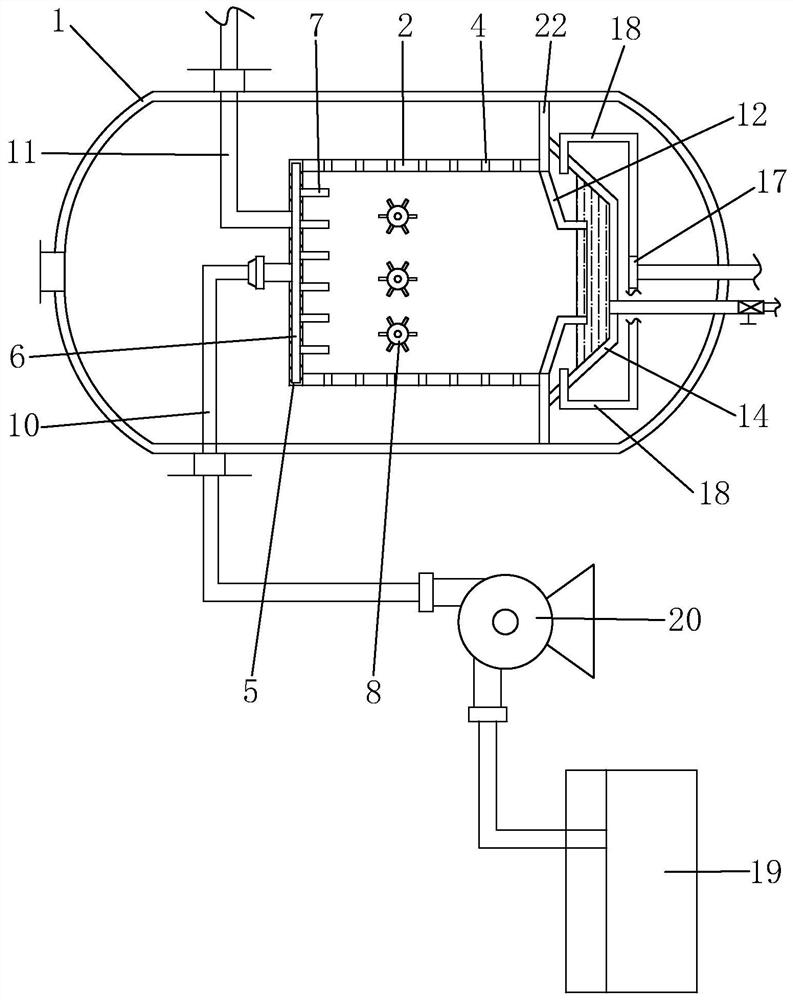
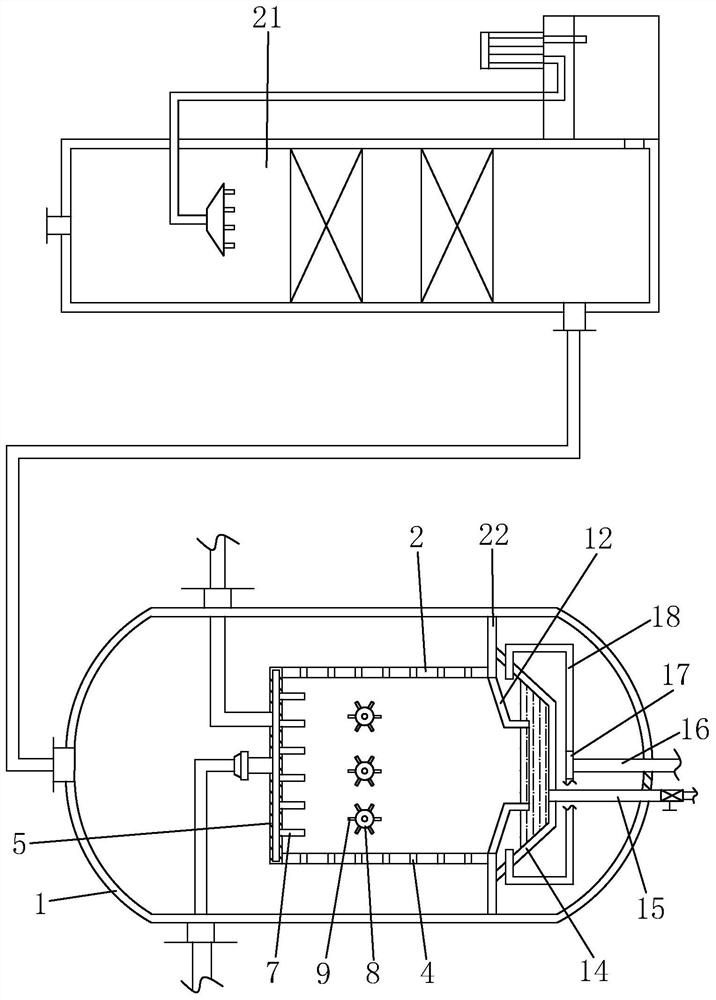
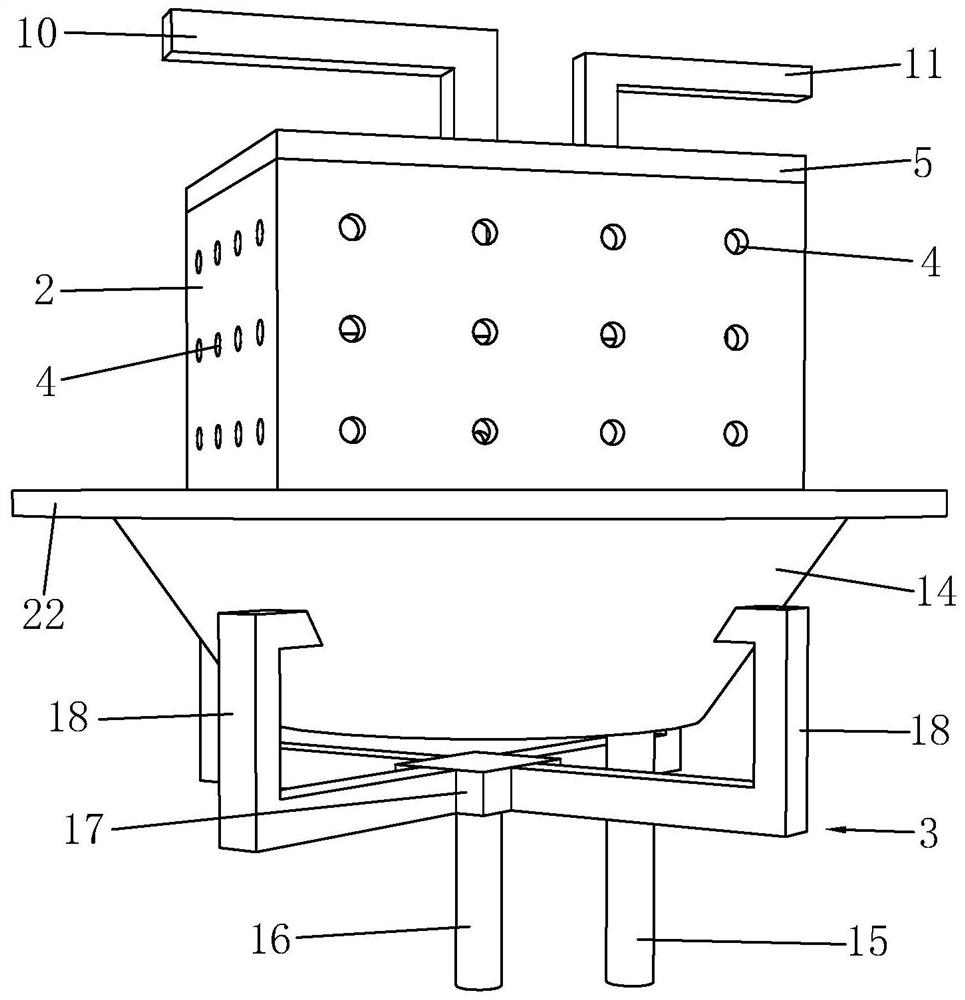
[0035] Such as Figure 1 to Figure 5 Shown, be the preparation method of a kind of bisfluorosulfonimide lithium of the present invention, comprise the steps:
[0036] (a) Condensation reaction: first purify chlorosulfonic acid, the boiling point of chlorosulfonic acid is 151°C, use the principle of atmospheric distillation, use still kettle to distill chlorosulfonic acid, collect the fraction with boiling point at 150~152°C, generally Chlorosulfonic acid sold on the market contains a small amount of HCl, SO 3 , SO 2 , Cl 2 , SO 2 Cl 2 Impurities such as impurities are purified before use, which improves the purity of chlorosulfonic acid and reduces the impact of these impurities on subsequent reactions. Then add chlorosulfonic acid and catalyst into the reaction kettle, stir and heat up to 100-120°C, then add chlorosulfonyl isocyanate dropwise for reaction, the molar ratio of chlorosulfonic acid to chlorosulfonyl isocyanate is 1:0.9-1: 1.1, to obtain dichlorosulfonimide,...
Embodiment 1
[0052] 450g of chlorosulfonic acid and 1.8g of ZnCl 2Add it into the reaction kettle, start stirring, heat up to 100-105°C, add 630g of chlorosulfonyl isocyanate dropwise for reaction, stir for 9 hours, and distill under reduced pressure to obtain 813g of the product dichlorosulfonyl imide; take 680g of dichlorosulfonyl isocyanate Imine, 0.6gSnCl 4 Put it into the reaction tower 1, raise the temperature to 95-100°C, pass 160g of hydrogen fluoride into the reaction tower 1, react for 7 hours, cool down to room temperature naturally, and pass in nitrogen for 6 hours to obtain 561g of the product bisfluorosulfonimide; 500g of acetic acid Methyl ester, 10gLiOH·H 2 Add O into the reactor, lower the temperature to 0-5°C, add 150g of bisfluorosulfonimide under stirring, filter after the reaction, and precipitate the filtrate under reduced pressure to obtain viscous lithium salt of bisfluorosulfonimide , add the above-mentioned bisfluorosulfonyl imide lithium salt into a dissolving ...
Embodiment 2
[0054] 500g of chlorosulfonic acid and 2.0g of MnCl 2 Add it into the reaction kettle, start stirring, heat up to 100-105°C, add 700g of chlorosulfonyl isocyanate dropwise for reaction, stir for 10 hours, and distill under reduced pressure to obtain 946g of the product dichlorosulfonyl imide; take 800g of dichlorosulfonyl isocyanate imine, 1.2g SbCl 5 Put it into the reaction tower 1, raise the temperature to 95-100°C, pass 220g of hydrogen fluoride into the reaction tower 1, react for 8 hours, cool to room temperature naturally, and pass nitrogen for 7 hours to obtain 673g of the product bisfluorosulfonimide; 500g of carbonic acid Dimethyl ester, 34gLi 2 CO 3 Add it into the reactor, lower the temperature to 0-5°C, add 150g of bisfluorosulfonimide under stirring, filter after the reaction, and desolvate the filtrate under reduced pressure to obtain viscous lithium salt of bisfluorosulfonimide. Add the above-mentioned lithium bisfluorosulfonyl imide salt into a dissolving t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com