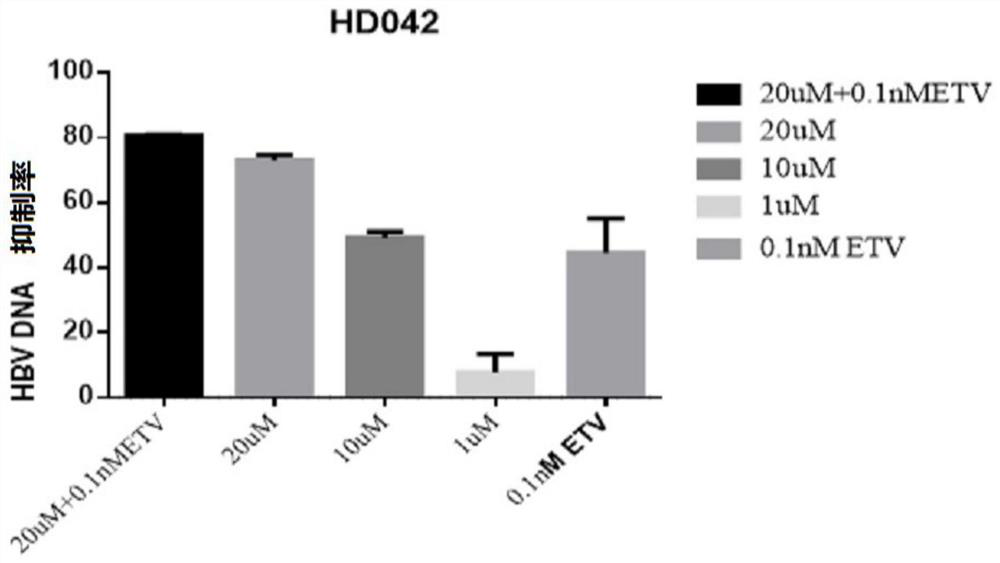
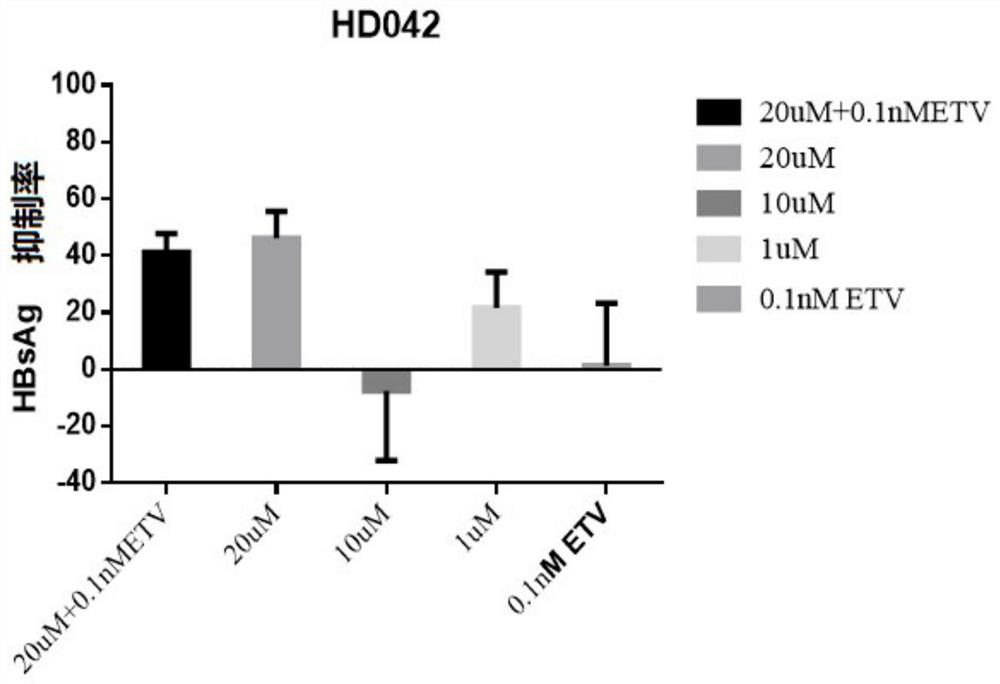
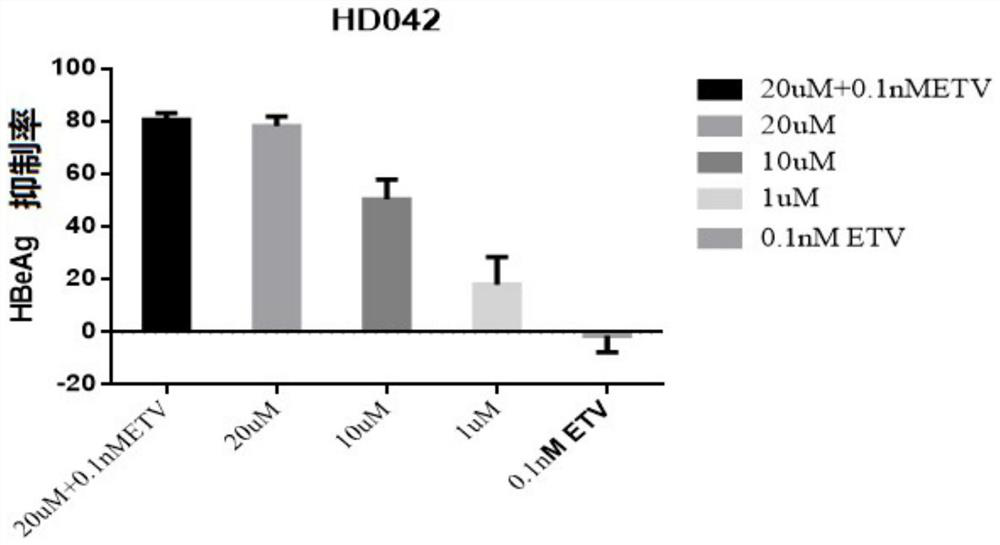
Application of a compound in the preparation of medicines for treating or preventing viral hepatitis
A compound and drug technology, applied in antiviral agents, pharmaceutical formulations, organic active ingredients, etc., to achieve excellent clinical safety, good druggability, and the effect of curing hepatitis B
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1-application of HepG2-NTCP cells to evaluate the in vitro anti-HBV activity of the compound of formula 1
[0086] Compound preparation method is as follows:
[0087] Take the preparation of a stock solution with a concentration of 20mM as an example, the volume of the solvent DMSO (μl) = sample mass (mg) × purity ÷ molecular weight ÷ 20 × 10 6
[0088] Control compounds include ETV (lot number: P1214012; 99.0% purity), purchased from Shanghai Titan Technology Co., Ltd. The positive control compound RG7834 (batch number: ET25747-14-P1; 99.5% purity) was purchased from Shanghai WuXi Kangde New Drug Development Co., Ltd.
[0089] The stock solutions of the above reference compounds were all at a concentration of 20 mM and stored at -20°C.
[0090] Table 1. Major Reagents and Cell Viruses
[0091]
[0092]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com