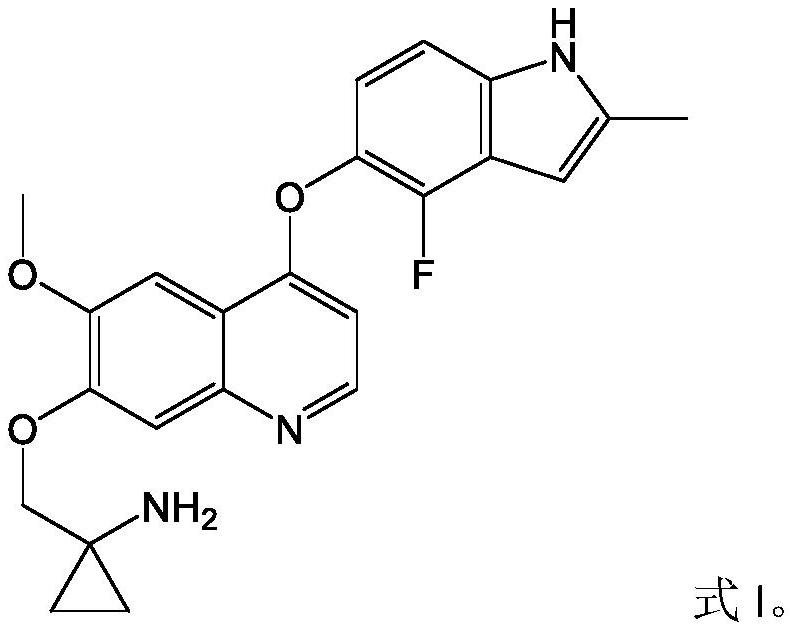
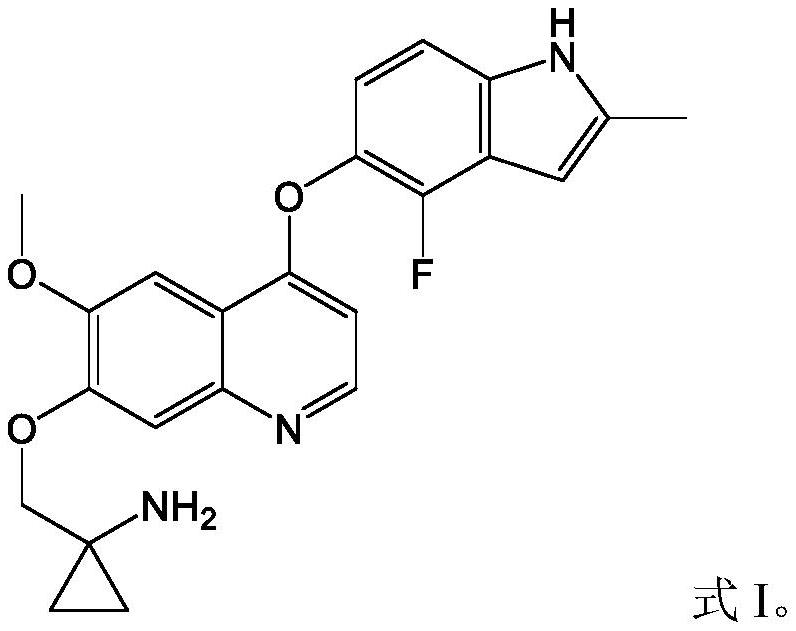
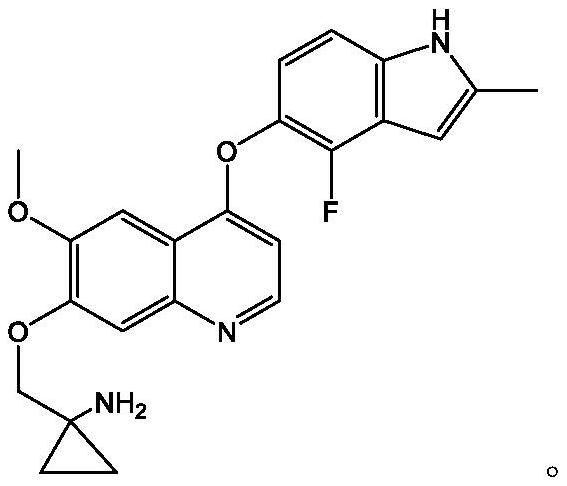
Pharmaceutical combination of quinoline derivative and PD-1 monoclonal antibody
A PD-1 and drug technology, applied in the field of medicine, can solve problems such as poor curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Embodiment 1 clinical research scheme-research standard and end point
[0143] 1.1 Inclusion and exclusion criteria
[0144] Inclusion criteria: Those who meet the following items can be included in this trial
[0145] 1) Meet all the conditions of any of the following cohorts:
[0146] Queue one:
[0147] a) Histopathologically confirmed unresectable or metastatic biliary tract cancer subjects, including intrahepatic cholangiocarcinoma (IHCC), extrahepatic cholangiocarcinoma (EHCC) and gallbladder cancer (GBC);
[0148] b) Previously failed first-line or above-line chemotherapy. The failure of first-line or more chemotherapy was defined as: disease progression during or after the last treatment; or intolerable due to toxic side effects during treatment.
[0149] Queue two:
[0150] Histopathologically confirmed recurrent or metastatic colorectal cancer with MSI-H or dMMR not suitable for surgical resection. No previous systemic therapy for recurrent or metastatic...
Embodiment 2
[0194] Embodiment 2. Clinical trial design
[0195] A single-arm, open, multi-cohort, multi-center phase II clinical trial was adopted.
[0196] 2.1 Sample size
[0197] There are 5 cohorts in this study, and 20-30 cases are enrolled in each cohort (adjusted according to the specific test results).
[0198] 2.2 Image Evaluation Design
[0199] The main efficacy endpoint of this study is ORR, which is evaluated by investigators of each research center. In this study, an independent imaging team was set up to carry out the review of imaging efficacy evaluation.
[0200] 2.3 Dosing regimen design
[0201] The administration objects are all patients recruited from cohort 1 to cohort 5 in Example 1.
[0202] The research was divided into two phases:
[0203] Phase 1 is a single-arm study with a safety lead-in period.
[0204] Patients will receive anlotinib combined with 14C12H1L1 therapy. Every 21 days is a treatment cycle, and safety information is collected to determine ...
Embodiment 3
[0235] Example 3. Collection of Biological Samples
[0236] 3.1 Detection of serum anti-14C12H1L1 antibody (ADA)
[0237] The time point of immunogenicity monitoring is based on the administration time of 14C12H1L1 injection; when the administration of 14C12H1L1 is delayed, blood collection for immunogenicity is delayed accordingly. If it is detected that the subject's ADA is positive, the neutralizing antibody will be tested additionally.
[0238] Collected before administration (-60min) in the 1st, 2nd, 4th, 8th cycle and every 6th cycle thereafter. At the same time, it was collected 30 minutes (±5 minutes) after the infusion of the first cycle and the eighth cycle, and 30 days (±7 days) and 90 days (±7 days) after the last administration. It is necessary to collect 5mL of venous blood each time, put it in a blood collection tube containing coagulation-promoting separation gel, place it at room temperature for 30 minutes, centrifuge it at 3000g for 10 minutes after natural...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com