Method for recovering high-content natural d-alpha-tocopherol succinate from leftovers
A tocopherol succinate, high-content technology, applied in organic chemistry, fermentation and other directions, can solve the waste of resources, the inability to propose α-tocopherol succinate crystallization methods, and limit the crystallization yield of crystalline tocopherol succinate, etc. problems, to achieve the effect of good industrialization feasibility, strong operability, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
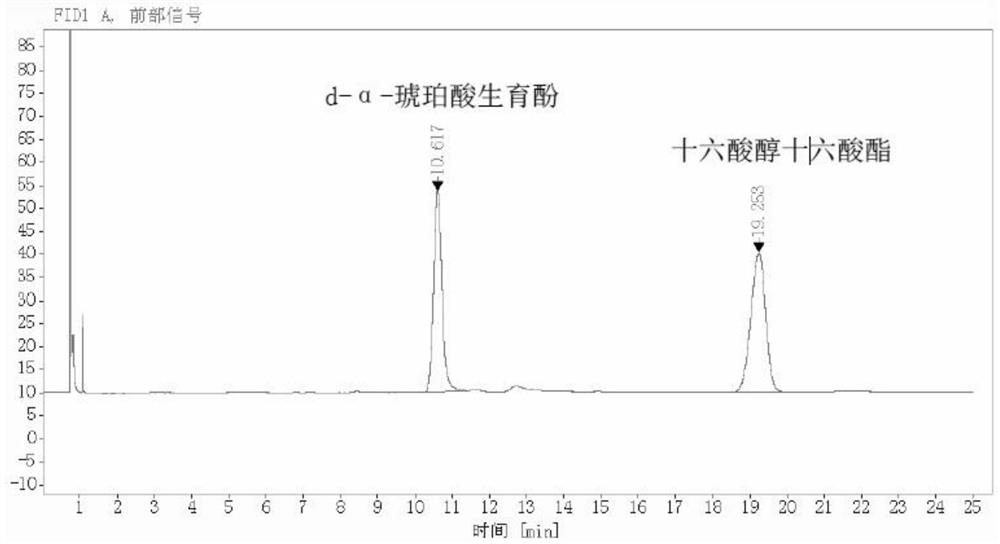
[0034] A method of recovering high content natural D-α-gyphenol succinate from the foot, comprising the step of: taking 1.5 kg of foot (D-α-tocapol succinate content of 28.5%), Heat 42 ° C, after filtration of 200 macotte filtration, 7.5 kg of methanol was added to the esterifying kettle, and the heat was stirred to dissolve the feet, and 30 g of concentrated sulfuric acid was added dropwise, and the dropwax time was 40 min, and the temperature was 40 min. The reaction was reacted at 68 ° C for 3.5 h. The reaction was completed, and the methanol was evaporated under reduced pressure at 75 ° C, and the methanol was used to the temporary tank for use, and the water was washed with a hot water at 70 ° C after steaming until there was no methanol. Wash to wash to pH> 6. After washing, the water was washed into the molecular distillation system, first performed the first stage film distillation, dehydration treatment at 140 ° C, continuously carrying the second stage short range distil...
Embodiment 2
[0037] A method of recovering high content natural D-α-gyphenol succinate from the foot, comprising the step of: taking 1.5 kg of foot (D-α-tocapol succinate content is 22.7%), Heat 45 ° C, after filtration of 200 martial filters, 9 kg of methanol was added to the esterification, and the heat was stirred to dissolve the foot, and 45 g of concentrated sulfuric acid was added dropwise, and the dropwise time was 50 min, and the temperature was 10,000. ° C reaction for 4 h. The reaction was completed, and the methanol was evaporated under reduced pressure at 73 ° C, and the methanol was used to the temporary tank for use, and the water was washed with 65 ° C for methanol. Wash to wash to pH> 6. The water was washed into the molecular distillation system, first performed the first grade film distillation, dehydration treatment at 150 ° C, continuously carrying the second stage short range distillation, distilled at 175 ° C to the resulting methyl ester product, weight 353.2g.
[0038]T...
Embodiment 3
[0040] The present invention recovered a method of recovering high content natural D-α-tocobquinate from the foot, including the steps of: taking 1.5 kg foot (D-α-tocophenous succinate content of 34.2%), Heat 44 ° C, after filtration of 200 macotte filtration, 10.5 kg of methanol was added to the esterifying kettle, and the heat was stirred to dissolve the foot, and 60 g of concentrated sulfuric acid was added dropwise, and the dropwax time was 60 min, and the temperature was heated. The reaction was reacted at 690 ° C for 4 h. The reaction was completed, and the methanol was evaporated under reduced pressure at 78 ° C, and methanol was hit to the temporary tank for use, and the water was washed with 73 ° C after stirring, water was washed to pH> 6. The water was washed into the molecular distillation system, first performed the first stage film distillation, dehydrated at 145 ° C, continuously subjected to the second stage short range distillation, distilled at 165 ° C to the obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com