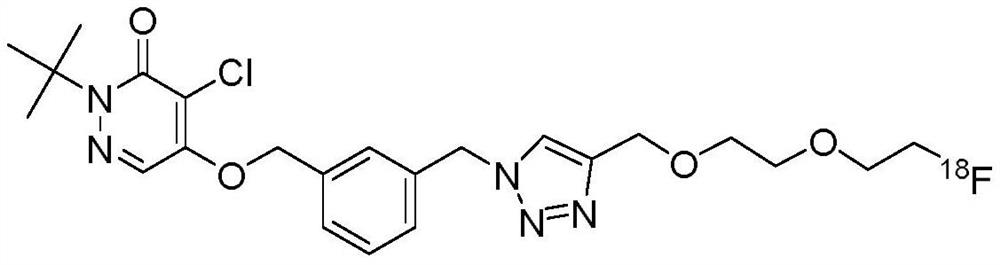
Preparation method and application of pyridazinone myocardial perfusion PET radiopharmaceutical
A technology of radiopharmaceuticals and myocardial perfusion, which is applied in the direction of radioactive preparations, radioactive carriers, and pharmaceutical formulations in vivo, and can solve the problems of unclear process flow, unclear applicability of large-scale activity production, and low demand for Fmpp2 activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
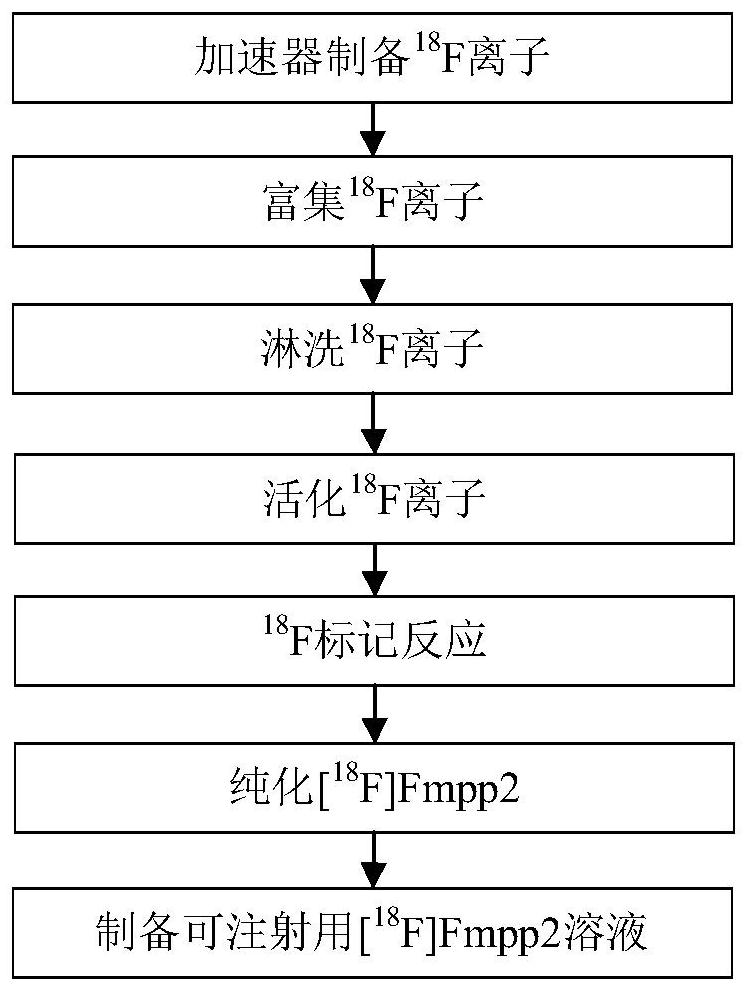
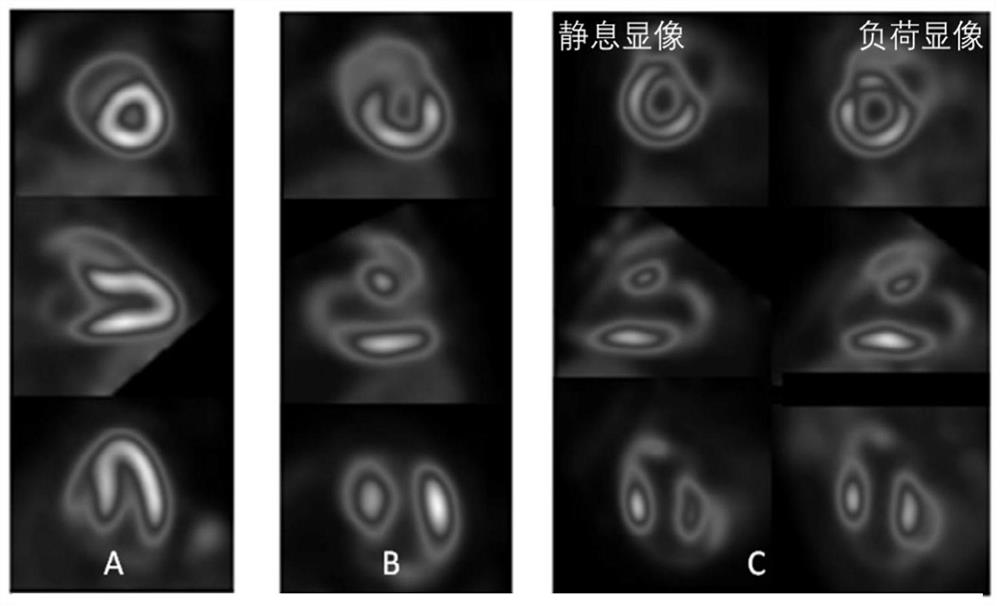
Image
Examples
Embodiment 1
[0040] The automatic equipment selects the equipment of model AllinOne of Trasis Company. The power unit of the equipment is high-purity nitrogen and injector electric rotor, which can provide a vacuum system and is equipped with an HPLC purification system. Because this process uses automation equipment, the automation equipment is placed in a radiation shielding box, which can protect the operator from radiation damage and increase the operating dose. At the same time, due to the control of the computer, the control of the process steps can be more precise and repeatable. Reduce human bias.
[0041] 1) The existing technology generally adopts 10-50mCi 18 F initial dose, the present embodiment adopts large dose initial 18 F, the preferred initial activity is 800-1000mCi.
[0042] 2) Enrichment 18 F ion anion exchange column choose Waters brand QMA column, QMA column preferably 1mol / L NaHCO 3 activation.
[0043] 3) Using cryptane K 222 with K 2 CO 3 solution rinse, e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com